Aggarwal Reviews the Latest Data Related to Systemic Therapy in Stage III NSCLC
In a Targeted Oncology case-based peer perspectives live discussion, Charu Aggarwal, MD, MPH, discussed systemic treatment options for stage III non–small cell lung cancer, based on a real case of a 63-year-old male patient.

In a Targeted OncologyTM case-based peer perspectives live discussion, Charu Aggarwal, MD, MPH, Leslye M. Heisler assistant professor for Lung Cancer Excellence, University of Pennsylvania Abramson Cancer Center, discussed systemic treatment options for stage III non–small cell lung cancer, based on a real case of a 63-year-old male patient.
Case
History and physical exam
• A 63-year-old man presented to his primary care physician with intermittent cough and difficulty breathing on exertion.
• Medical history: hyperlipidemia, well managed on simvastatin; hypothyroidism, managed on
levothyroxine; chronic obstructive pulmonary disorder, managed on inhalers
• Tobacco: recently quit smoking; 40-pack-year history
• Physical exam: intermittent wheezing; otherwise negative
• ECOG performance status: 1
• Labs: complete blood count, chemistries, and creatinine all within normal limits
• Chest CT: 3.1-cm spiculated left upper lung mass; 2 enlarged left mediastinal lymph nodes measuring 2.5 cm and 1.7 cm; moderate emphysema
• PET scan: confirmed lung lesion and mediastinal lymphadenopathy without evidence of distant metastases
• Brain MRI: negative
• Pulmonary function tests:
- Forced expiratory volume in 1 second: 1.2 L
- Diffusing capacity for carbon monoxide: 35%
• Bronchoscopy with transbronchial lung biopsy of left upper lung mass and lymph node sampling via endobronchial ultrasound revealed adenocarcinoma at the primary site with positive nodes in stations 4L and 7; level 4R was negative.
• Staging: T2aN2M0, stage IIIA
• Based on the bulk and extent of mediastinal disease and active emphysema, the patient’s cancer was deemed unresectable and he was referred for consideration of concurrent chemotherapy and radiation.
TARGETED ONCOLOGY™: What are the options for frontline therapy in this patient?
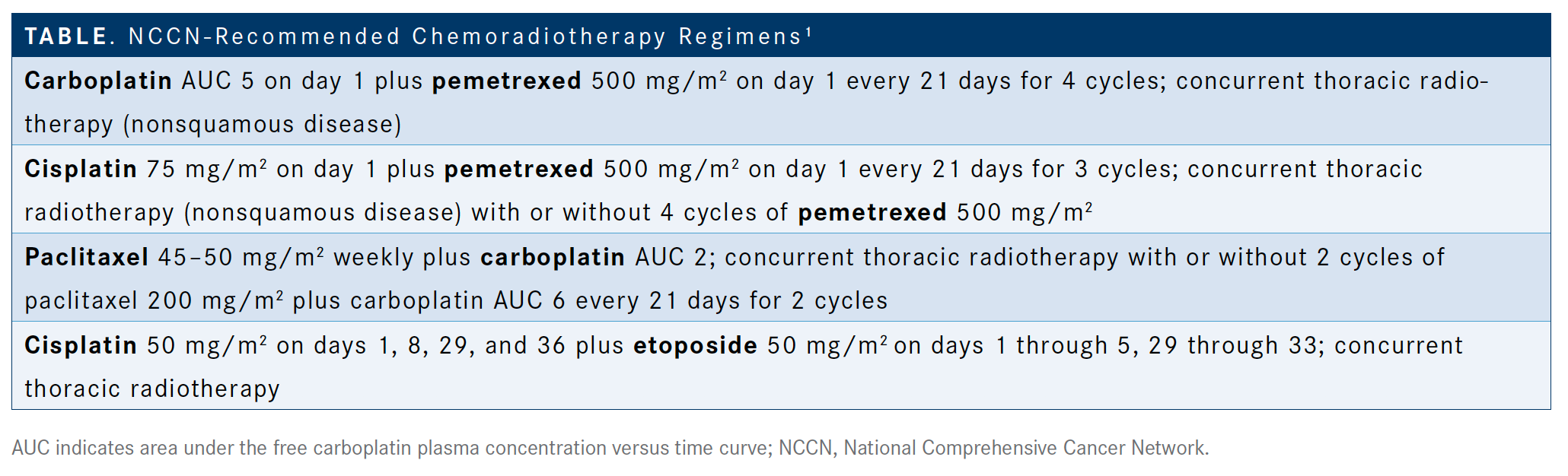
Aggarwal: Almost all of our cases at Penn [Medicine] are for at least stage III disease [and] are discussed at tumor boards. Our surgeons take the first steps in calling patients resectable versus unresectable. Once they are considered unresectable, we have been taking them through chemoradiation. What are your usual regimens for chemoradiation? I think [chemotherapy plus immunotherapy is] the practice. One of the questions that we have is [whether or not to] test for PD-L1 in stage III disease. The options for treatment for chemoradiation are listed [in the National Comprehensive Cancer Network (NCCN) guidelines],1 but we know this patient is unresectable. There’s cisplatin and etoposide with concurrent thoracic radiotherapy. Carboplatin plus pemetrexed is a viable alternative that is usually used for about 4 cycles. Then, there is cisplatin plus pemetrexed [Alimta] or carboplatin plus paclitaxel, which is the low-dose weekly regimen [TABLE].1
If there’s a young patient, about 50 years old, who was a smoker and I wanted to be as aggressive as possible, I would prefer to use a cisplatin/etoposide regimen. For most of the patients, I am using carboplatin and paclitaxel.
Case (continued)
• The patient underwent therapy with cisplatin plus pemetrexed and concurrent thoracic radiotherapy.
• Follow-up imaging showed a partial response (PR) with shrinkage of the primary and nodal lesions.
• There was no evidence of distant spread observed.
Could this patient benefit from further treatment? How do you follow this patient?
With the data on durvalumab [Imfinzi], I’m not using the consolidation chemotherapy as I was earlier. I am stopping chemotherapy at the time of radiation. I would usually stop at that point in time, get a CT scan in 2 weeks, and then start durvalumab. I’m not using these 2 cycles that we used to use because we didn’t have anything else at that time.
This patient underwent therapy with cisplatin and pemetrexed with concurrent thoracic radiation. Follow-up imaging showed a PR with shrinkage and that [there was] no evidence of distant disease spread. The CT scan is not [intended] to look for a response but is mainly
to make sure that they haven’t had progression. Two weeks would be too early to get a PET [scan]. I usually get a CT scan, sometimes even without intravenous [IV] contrast but mostly with it, to make sure that they haven’t progressed. If the patient hasn’t progressed, I start them on durvalumab.
I don’t have the durvalumab discussion at the time they come back and see me. I have a durvalumab discussion initially because I tell them that is a package…that they’re going to get chemoradiation.
They’re going to have a 2- or 3-week break, then they start durvalumab. It’s not a surprise to them. They’re almost grateful that they don’t have to come back weekly anymore because
[durvalumab administration] is every 2 weeks. In fact, new clinical trials are evaluating an every-4-weeks regimen of durvalumab, which would be even better.
Would you approach this patient differently if the performance status was 2 after chemoradiation therapy or if the patient had stage IIIC disease?
Some patients do get beaten up with chemoradiation. Some people get hospitalized and it can be toxic.
Data have shown that 14 days is the best interval to start immunotherapy. The usual timeline allows up to 42 days to start, [although] there have been some subgroup analyses that show that if you start within 14 days, you can have a better response.
Please describe the trial that led to the approval of durvalumab in this setting.
In the PACIFIC study [NCT02125461], patients with stage III, locally advanced unresectable lung cancer who had not progressed following definitive platinum-based chemoradiation therapy were randomized 1 to 42 days post chemoradiation…to receive either durvalumab for up to 12 months counting from the first day of infusion or placebo.2
The primary end point was progression-free survival [PFS]. Durvalumab showed a significant improvement in median PFS, almost a tripling. It was 5.6 months in the control arm versus
17.2 months with [durvalumab and] a hazard ratio of 0.51 [95% CI, 0.41-0.63], a dramatic improvement.2
We started using this right after the [data were released]. It wasn’t approved, but we were able to get durvalumab through AstraZeneca. When we looked at the meta-analysis, we saw that regardless of histology or disease status, the patients responded.
Two years ago, we heard PD-L1 status may have something to do with response. People that were negative for PD-L1 expression may not respond that well.3 Then, EGFR mutational status was another factor for which the hazard ratio for PFS crossed 1.0.2 EGFR-mutant tumors did not seem to benefit from use of immunotherapy in this setting.
[By PFS subgroup analysis], if radiation therapy had been done within 14 days of initiating durvalumab, the benefit was even higher, with [a] hazard ratio of 0.39 [95% CI, 0.26-0.58] versus 0.63 [95% CI, 0.49-0.80] for patients who started more than 2 weeks after radiation.2 That’s the reason why I try and get patient[s] on therapy early.
Recently, updated overall survival [OS] data from PACIFIC [were] published. The OS rate for durvalumab at 3 years is 57.0% compared with 43.5% for placebo alone. There is definitely a [separation] to the Kaplan-Meier curve for survival that’s continuing to mature and develop here.4
If you look at the new lesions that are developing, most of these are developing less frequently on the durvalumab arm compared with the placebo arm. The overall rate of new lesions at any site was 22.5% versus 33.8%.4 Durvalumab doesn’t obviate the development of metastatic disease but it certainly reduces it.
Please discuss the analyses based on PD-L1 expression status showing that certain patients may not benefit from the addition of this therapy.
There are some caveats to these data. There was a post hoc analysis. Currently in the United States, the PACIFIC regimen is approved for all histologies regardless of PD-L1 status. Fewer
than 60% of patients on the trial had adequate tissue for PD-L1 assessment. There were small numbers in the PD-L1 subgroups, with only 148 patients with <1% expression. [These data] are not something that we can base our entire practice on.3
I give patients durvalumab in my practice who are negative for PD-L1. At 3 years, the hazard ratio for negative PD-L1 expression had dropped to 1.14 [from 1.36 observed in the initial OS data]. These are all hypothesis-generating data.
In Europe, durvalumab is only approved for tumor[s] with PD-L1 expression ≥1%. I think that’s more of a cost-effectiveness determination. They’re much more restrictive and I certainly see efficacy within my patients with PD-L1–negative disease.
What would you do for a patient who has an EGFR mutation?
Recently, I had 2 or 3 patients that had EGFR mutations and stage III non–small cell lung cancer [NSCLC]. It’s a tricky question because on one hand, this is the curative regimen. My goal is for patients to receive this for 1 year and never have to have any other treatment again.
But again, this is lung cancer. If I give them durvalumab and then they develop metastatic disease, giving them osimertinib [Tagrisso] later predisposes them to toxicity. I think it’s a very tricky situation. I discuss this with patients. I will point out that there are a couple trials going on in this space.
The LAURA trial [NCT03521154], which is a friend of the phase III FLAURA trial [NCT02296125], is [evaluating osimertinib in] locally advanced disease…[and] is ongoing and almost completely accrued. It’s looking at patients with stage III NSCLC and EGFR mutations. Patients get osimertinib compared with placebo. I would be interested in treating these patients with osimertinib because I do think that they’ll benefit in terms of PFS and perhaps OS. We don’t know.
What is the toxicity profile like for durvalumab?
Overall safety is good. AEs [adverse events] look similar to placebo. All-cause AEs are 96.8% compared with 94.9%. Nothing jumps out at us [besides] perhaps more treatment-related AEs.
Pneumonitis is one of the main things that I get asked about and this is something that can get complicated, especially for patients with radiation therapy.
Case (continued)
• The patient was started on durvalumab.
• After cycle 8, he developed fatigue, mild shortness of breath, and mild cough without chest pain.
• He had no fever, no recent sick contact, and his flu vaccine was up-to-date.
• A chest CT was ordered at approximately week 16 to assess the patient’s symptoms.
If you’re concerned that this may be pneumonitis, what is your usual practice?
I usually look at the CT scan in terms of is this unilateral or is this bilateral. The things that I use to differentiate whether this is radiation-related pneumonitis or immunotherapy-related
pneumonitis is the radiation field itself because many of our patients have radiation-related pneumonitis. They’ll have it usually within the radiation field.
It usually presents 6 to 12 weeks out from radiation therapy and then the patient has features or symptoms that are indistinguishable from immune-related pneumonitis. Treatment for both is steroids. If it’s radiation-related pneumonitis, I’m more likely to taper their steroids quickly and get them back on immunotherapy. If it’s bilateral pneumonitis and I’m finding…they’re requiring oxygen, I usually wait for them to come to a point where they’re not requiring oxygen anymore before restarting [therapy].
Reference
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Non–Small Cell Lung Cancer (version 3.2020).nccn.org/professionals/physician_gls/pdf/nscl.pdf. Published February 11, 2020. Accessed April 1, 2020.
- Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937.
- Gray JE, Villegas AE, Daniel DB, et al. Three-year overall survival update from the PACIFIC trial. J Clin Oncol. 2019;37(suppl 15; abstr 8526). doi: 10.1200/JCO.2019.37.15_suppl.8526.
- Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.