The Revival of Vaccines in Lung Cancer
This article provides a short historical overview of major lung cancer vaccination studies performed in the last decade to set the perspective on the development of current clinical trials of therapeutic cancer vaccines for patients with non–small cell lung cancer.
Grace Dy, MD,
ABSTRACT
The renewed interest in therapeutic cancer vaccine approaches in lung cancer arises from the convergence of several key developments: the clinical success of immune checkpoint inhibitors, a better understanding of the role of immune checkpoints in the cancer immunity cycle in modulating the efficacy of cancer vaccines, and advancements in computational and bioinformatics platforms that enable the development of cancer neoantigen vaccination strategies. This article provides a short historical overview of major lung cancer vaccination studies performed in the last decade to set the perspective on the development of current clinical trials of therapeutic cancer vaccines for patients with nonsmall cell lung cancer.
As the title of this article implies, the recent resurgence of interest in the development of therapeutic vaccines in lung cancer therapy was preceded by a brief lull of apathy and even disdain for this approach. These opinions arose upon the successive lack of positive signals of several large, randomized, placebo-controlled, phase II/III trials of lung cancer vaccine studies, MAGRIT, START, and STOP, which began accrual more than a decade ago. In this review, we provide highlights in the clinical development of lung cancer vaccines from the historical perspective of these and other studies (Figure) and discuss new directions in cancer vaccine strategies for the treatment of lung cancer.
MAGRIT, a phase III study, investigated the efficacy of recombinant melanoma-associated antigen 3 (MAGE-A3) protein with AS15 (a combination of QS21, monophosphoryl lipid A, and CpG7909, a TLR-9 agonist, in a liposomal formulation) as an immunostimulant versus placebo in the adjuvant setting. It was administered over 27 months in patients with stage Ib, II, and III MAGE-A3positive non–small-cell lung cancer (NSCLC).1MAGE-A3 is a tumor-associated cancer-testis antigen that is differentially expressed in 30% to 50% of NSCLCs; it is not expressed in normal adult human tissues except the placenta and testis. MAGRIT was launched soon after the results of a randomized, placebo-controlled phase II study demonstrated a trend toward disease-free survival (DFS) and overall survival (OS) with the MAGE-A3 vaccine that was similar to what may be achieved with adjuvant chemotherapy (adjuvant chemotherapy at that time was not standard of care), although the results were not statistically significant.2 In MAGRIT, between 2007 to 2012, approximately 33% of the 12,820 patients with a valid sample submitted were determined to have a MAGE-A3positive tumor prior to study enrollment. A total of 2272 of the 2312 randomized patients received at least 1 study treatment injection. While the safety profile was favorable, there were no observed differences in the primary endpoints of DFS and OS between the experimental and control arms, whether or not patients received adjuvant chemotherapy.
Another disappointment was the development of tecemotide (L-BLP25), a liposomal peptide vaccine targeting the aberrantly glycosylated mucin 1 (MUC1) glycoprotein overexpressed in NSCLC, particularly in the nonsquamous subgroup. Small phase II trials in advanced-stage NSCLC showed a potential survival benefit in stage IIIb patients; this provided the rationale for the START trial, which investigated tecemotide versus placebo as maintenance therapy after completion of chemoradiation in 1239 patients with unresectable stage III NSCLC. The primary endpoint of OS was not met, with no significant difference between patients. Preplanned subgroup analyses showed that the subgroup of patients who received concurrent chemoradiotherapy appeared to demonstrate a statistically significant improvement in survival, whereas no difference in survival between treatment groups was observed in patients who received sequential chemoradiotherapy.3This provided justification to initiate START2 in April 2014, exploring tecemotide specifically in patients with unresectable stage III NSCLC who had a response or stable disease after platinum-based concurrent chemoradiation. However, clinical development of tecemotide was discontinued upon the analysis of a smaller trial conducted in Japanese patients, the majority of whom received concurrent chemoradiation. This study showed no apparent difference observed for any of the primary or secondary survival endpoints between the treatment groups.4Therefore, both START2 and INSPIRE (a phase III study enrolling Asian patients) terminated accrual in September 2014.
The fate of belagenpumatucel-L (Lucanix) was similarly doomed. It was an allogeneic vaccine derived from 4 NSCLC cell lines transfected with a human transforming growth factorB2-antisense plasmid vector to increase its immunogenicity. Dose-dependent survival difference was seen in a small randomized, dose-finding phase II trial enrolling mostly patients with stage IV NSCLC.5This provided the rationale for the STOP trial, which evaluated belagenpumatucel-L versus placebo as maintenance therapy for up to 20 doses in 532 patients with response to or stable disease after platinum-based first-line chemotherapy.6The primary endpoint of OS was not met in the intention-to-treat analysis. A prespecified Cox regression analysis demonstrated that patients randomized to the vaccine arm within 12 weeks of completion of chemotherapy or those who had received prior radiation appeared to have longer survival compared with the placebo group. Clinical development for these 3 vaccines have all been discontinued.
Even before the results of MAGRIT, START, and STOP trials were presented between 2013 and 2014, the oncology field had already been experiencing a renaissance in cancer immunotherapy, as interest grew with the release of early findings from clinical trials of adoptive cell therapy and immune checkpoint inhibitors.7-9This shifted attention away from other vaccination studies subsequently reported, the results of which showed early signs of potential clinical benefit with these strategies.
Racotumomab is an anti-idiotype monoclonal antibody against ganglioside containing a Neu glycolyl (NeuGc), specifically NeuGcGM3. Anti-idiotypic antibodies mimic the nominal antigen and thus can elicit an immunogenic reaction against the original epitope of the selected antigen. NeuGcGM3 ganglioside is differentially expressed in various malignancies, including NSCLC.10,11A randomized, phase II, double-blinded trial of racotumomab (using alum as adjuvant) versus placebo as switch maintenance treatment after response to or stable disease after first-line chemotherapy enrolled 176 patients between 2006 and 2010 in Cuba, where this agent was developed. Median progression-free survival (PFS) and OS in the vaccinated patients were better compared with placebo (PFS: 5.3 vs 3.9 months; hazard ratio [HR], 0.73; P = .039; OS: 8.2 vs 6.8 months; HR, 0.63; P = .004).12 A larger, international, randomized phase III trial (NCT01460472) of racotumomab versus best supportive care in the same treatment setting for NSCLC was subsequently begun in 2010; accrual of about 1080 patients in South America and Southeast Asia was completed in September 2016. Final results are awaited.
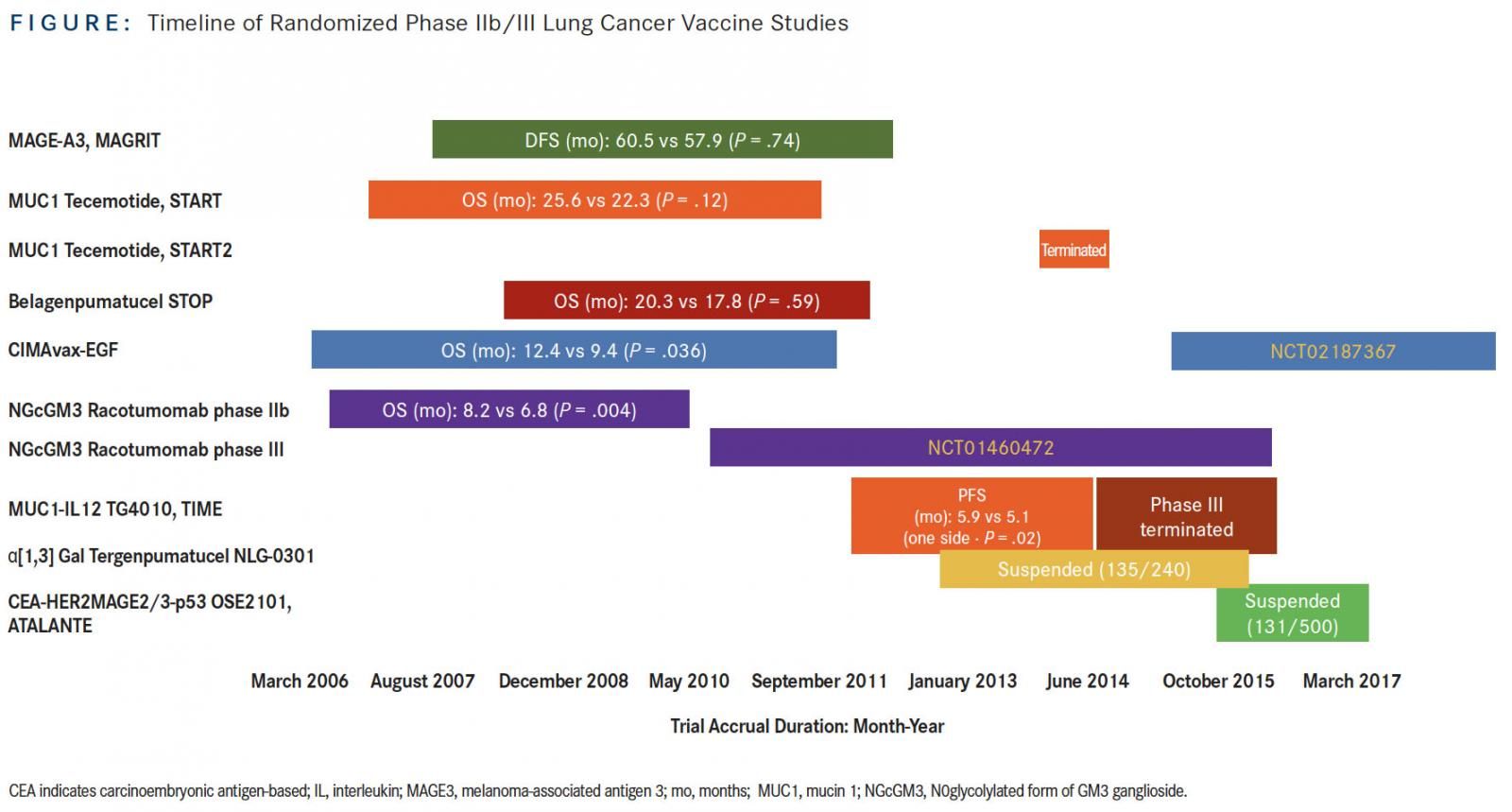
CIMAvax-EGF is a vaccine composed of recombinant human EGF chemically conjugated to a recombinant carrier protein, p64, from Neisseria meningitidis, using montanide as the adjuvant. Initial studies of CIMAvax-EGF began in the 1990s, culminating in a randomized phase III trial conducted in Cuba between 2006 and 2012. A total of 405 patients with advanced NSCLC were randomized to receive either CIMAvax-EGF or best supportive care as switch maintenance after response to or stable disease after platinum-based chemotherapy. Final results showed that OS was numerically higher in the vaccine versus control arm in the safety population (receiving at least 1 dose of vaccine), with an HR of 0.82 (P = .1). In the landmark analysis of per-protocol population consisting of patients who received at least 4 vaccine doses (for the control group, patients not surviving for the same period of time were excluded), median OS was 12.4 versus 9.4 months in the control group (HR, 0.77; P = .036). The survival effect was even more striking for patients who had high EGF concentration (serum EGF >870 pg/ml) at baseline: Median OS for vaccinated patients in this group was 14.7 versus 8.6 months in the matched control group. The interaction between EGF levels and treatment was statistically significant (P <.0001). Conversely, patients who mounted good antibody titers after the induction period similarly had a significant survival benefit compared with the control group.13The relationship between antibody levels and EGF levels is inversesurvival is better with lower EGF than higher EGF at baseline, and better survival was demonstrated with those patients who ultimately developed higher antibody levels. An ongoing randomized international phase III study of CIMAvax-EGF in combination with first-line platinum-based chemotherapy is for patients with stage IV NSCLC; baseline EGF level is a biomarker for selection, with a cut-off of >250 pg/ ml. This cut-off is based upon retrospective analysis of samples from the 405-patient phase III Cuban trial, which showed correlation with clinical efficacy at this threshold level.14
TG4010 is a viral-based vaccine consisting of recombinant Modified Vaccinia Ankara virus encoding MUC1 and interleukin 2. Between 2012 and 2014, 222 patients were enrolled into a randomized, double-blind, placebo-controlled, trial called TIME, designed as a phase IIb/III study to evaluate TG4010 in combination with first-line chemotherapy in advanced NSCLC. The study met its primary endpoint of PFS (5.9 vs 5.1 months; HR, 0.74; P = .019). Prespecified subgroup analysis of the prospective biomarker validation of the companion diagnostic as well as histology was performed. The triple-positive activated lymphocyte (TrPAL) biomarker referred to circulating percentage of lymphocytes with the natural killer cell signature (CD16, CD56, CD69) as determined by flow cytometry. Efficacy analysis showed that patients with low TrPAL value (less than or equal to the third quartile) or nonsquamous histology exhibited significantly improved PFS and OS outcomes with vaccination compared with placebo, with the highest benefit in the subgroup of patients with both nonsquamous histology and low TrPAL value.15 These results were part of the phase IIb portion of TIME, providing justification for further investigation of the agent.
However, a better understanding of the fundamental basis for the cancer-immunity cycle has emerged since then.16It is currently recognized that immune-suppressive factors exist in the tumor microenvironment. This local immunosuppression reflects a pathologic state co-opted from homeostatic mechanisms that arise from the interplay of cellular and metabolic functions to regulate inflammation. This may explain why vaccine-based therapies were previously ineffective. Better understanding of the cancer immunity cycle gave rise to recognition that local immunosuppression prevents vaccines from being effective, even though antibody response/cellular response can be elicited as other mechanisms exist to suppress efficacy of the elicited vaccine response, apart from issues related to the selection of adjuvants. Indeed, preclinical and clinical translational studies have shown that therapeutic cancer vaccination attempts can upregulate the PD-1/PD-L1 pathway17and thus mitigate the very effect that would be desired from vaccination.17,18Moreover, since 2015 immune checkpoint inhibitor therapy has changed the long-entrenched treatment algorithm; therefore, it is not surprising that cancer vaccines, along with virtually all other kinds of anti-cancer therapies, are currently being explored in clinical trials in combination with antiPD1/PD-L1 agents. For instance, a trial of CIMAvax-EGF in combination with nivolumab as second-line therapy for advanced NSCLC began recruitment in December 2016 (NCT02955290). In fact, this focus on checkpoint inhibitor therapy in the treatment landscape has led to the termination of the phase III portion of TIME, as new studies have been launched investigating the combination of TG4010 with nivolumab after failure of platinum-based chemotherapy (NCT02823990), and the combination of TG4010 with nivolumab and chemotherapy in the first-line setting in nonsquamous NSCLC (NCT03353675).19
Other vaccine studies in NSCLC that terminated accrual early or were suspended for adjustment in recruitment strategy include NLG-0301 and ATALANTE 1. NLG-0301 is a phase II/III study of tergenpumatucel versus docetaxel as second-line therapy in NSCLC. Tergenpumatucel is another cell-based vaccine, composed of 3 allogeneic NSCLC cell lines genetically modified to express the foreign carbohydrate α1,3 galactosyltransferase, which underscores the hyperacute rejection of foreign transplanted tissues. NLG-0301 closed further enrollment in June 2016, with 135 of 240 planned patients already accrued. Tergenpumatucel is currently being tested in a phase I/II study of combination with indoximod, which inhibits indoleamine 2,3-dioxygenase, and docetaxel in NSCLC. The randomized phase III ATALANTE 1 trial (NCT02654587), initiated in early 2016, investigates OSE2101 (Tedopi) versus docetaxel or pemetrexed as second- or third-line treatment in patients with HLA-A2positive metastatic NSCLC. OSE2101 is a DNA-based vaccine composed of 10 selected antigenic epitopes against 5 tumor-associated antigens (TAAs)carcinoembryonic antigen, HER2, MAGE2, MAGE3 and p53—and optimized for binding to HLA-A2–positive T cells.20The study’s accrual had been temporarily suspended for interim analysis in June 2017, but on December 7, 2017, the trial’s independent data monitoring committee provided conditional recommendation for its resumption. The committee recommended that accrual should now focus on recruiting patients who have failed prior therapy with anti-PD1/PD-L1 therapy, which would reflect broad clinical practice of the utilization of docetaxel after failure of immunotherapy in this population.
Another one of the posited central problems surrounding the previous failure of cancer vaccination approaches has been the selection of antigens, such as TAAs, to which there may be self-tolerance. Cryptic peptides are not typically involved in the self-tolerance process but ordinarily have low affinity for presentation by HLA-I molecules. Vx-001, therefore, is a peptide-based vaccine formulated to optimize the immunogenicity of the cryptic epitope derived from a universal tumor antigen, telomerase reverse transcriptase (TERT) for binding to HLA-A2positive T cells.21In a randomized, double-blind, placebo-controlled, phase IIb clinical trial enrolling 190 HLA-A2positive patients with metastatic or recurrent NSCLC as maintenance therapy after response to or stable disease after platinum-based first-line chemotherapy, there were no significant differences in OS between the groups treated with Vx-001 and placebo in the overall population.22However, 29% of patients who were able to develop a vaccine-specific immune response demonstrated longer OS compared with nonresponders (21 vs 13 months, P = .004). Subset analysis also demonstrated potentially better OS with vaccine compared with placebo among patients who were never-smokers or light smokers and had non-immunogenic tumors (as defined by proprietary diagnostic biomarker test utilized in the trial), with median OS of 20.2 versus 7.9 months, respectively (P = .0001). According to the developer of Vx-001, this finding underlies future plans of investigating Vx-001 in combination with immune checkpoint inhibitors in this particular population of patients with non-immunogenic NSCLC, who conventionally do not demonstrate benefit from immune checkpoint inhibitor therapy alone.23
An alternative approach that has recently generated attention and interest surrounds the targeting of tumor neoantigens that arise from somatic mutations; these neoantigens are not only tumor-specific but also are highly immunogenic, as they are not subject to the central tolerance mechanism. Indeed, the confluence of technologic advancements in genomics and bioinformatics and steep reduction in cost barriers has facilitated the feasibility of individualized vaccine approaches targeting tumor neoantigens. These individualized vaccines are loaded with multiple RNA- or peptide-based neoepitopes identified after tumor whole exome sequencing, with the final selection of candidate neopitopes based on predicted MHC Class I and/or II binding affinity for each patient.24,25Early clinical trial results of the neoantigen vaccine approach in melanoma patients demonstrated tumor responses in patients with metastatic disease that could be related to the vaccine therapy.25
Current and future issues related to this approach are not in short supply: They include, for instance, logistics (time from initial tumor specimen collection to completion of vaccine manufacturing for each patient may take 6 to 12 weeks) and acquired resistance mechanisms. Nonetheless, in just 2 years, numerous agreements worth billions of dollars have already been solidified in pursuing this approach, with several individualized neoantigen-based vaccines (NEO-PV-01, RO7198457, mRNA-4157) in combination with anti-PD1/ PD-L1 agents now actively recruiting patients (NCT02897765, NCT03289962, NCT03313778).
We are at an exciting crossroads in terms of therapeutic advancements for a population of patients who historically have poor survival. Multiple agents now represent complementary therapies to potentially augment the immune response in cancer vaccination strategies. A recent analysis of the global immune-oncology landscape shows that as of September 2017, more cancer vaccines than any other class of therapeutic agents are under clinical investigation, with more than 340 agents in clinical trials and another estimated 260 agents in preclinical and discovery stages.26In fact, we currently face a bottleneck in terms of availability of suitable patients for these studies. Collaboration across all stakeholders (industry, academia, government, patient groups) to achieve cross-trial coordination strategies, data sharing, improved trial design, and selection of patients will be required to facilitate the dissemination of successful cancer vaccination strategies to more patients sooner rather than later.
References:
- Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3- positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(6):822-835. doi: 10.1016/S1470-2045(16)00099-1.
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;31(19):2396-2403. doi: 10.1200/JCO.2012.43.7103.
- Butts C, Socinski MA, Mitchell PL, et al; START Trial Team. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59-68. doi: 10.1016/S1470-2045(13)70510-2.
- Katakami N, Hida T, Nokihara H, et al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer. 2017;105:23-30. doi: 10.1016/j.lungcan.2017.01.007.
- Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense genemodified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4721-4730.
- Giaccone G, Bazhenova LA, Nemunaitis J, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 2015;51(16):2321-2329. doi: 10.1016/j.ejca.2015.07.035.
- Topalian SL, Hodi FS, Brahmer SR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465. doi: 10.1056/NEJMoa1200694.
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725-733. doi: 10.1056/NEJMoa1103849.
- Irie A, Koyama S, Kozutsumi Y, et al. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273(25):15866-15871.
- van Cruijsen H, Ruiz MG, van der Valk P, et al. Tissue micro array analysis of ganglioside N-glycolyl GM3 expression and signal transducer and activator of transcription (STAT)-3 activation in relation to dendritic cell infiltration and microvessel density in non-small cell lung cancer. BMC Cancer. 2009;9:180. doi: 10.1186/1471-2407-9-180.
- Alfonso S, Valdés-Zayas A, Snatiesteban ER, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2014;20(14):3660-3671. doi: 10.1158/1078-0432.CCR-13-1674.
- Rodriguez PC, Popa X, Martinez O, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22(15):3782-3790. doi: 10.1158/1078-0432.CCR-15-0855.
- Rosell R, Neninger E, Nicolson M, et al. Pathway targeted immunotherapy: rationale and evidence of durable clinical responses with a novel, EGF-directed agent for advanced NSCLC. J Thorac Oncol. 2016;11(11):1954-1961. doi: 10.1016/j.jtho.2016.08.132.
- Quoix E, Lena H, Losonczy G, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212-223. doi: 10.1016/S1470-2045(15)00483-0.
- Chen DS, Mellman I. Oncology meets immunology: the cancer immunity cycle. Immunity. 2013;39(1):1-10. doi: 10.1016/j.immuni.2013.07.012.
- Lutz Er, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616-631. doi: 10.1158/2326-6066.CIR-14-0027.
- Remy-Ziller C, Thioudellet C, Hortelano J, et al. Sequential administration of MVAbased vaccines and PD-1/PD-L1-blocking antibodies confers measurable benefits on tumor growth and survival: preclinical studies with MVA-βGal and MVA-MUC1 (TG4010) in a murine tumor model. Hum Vaccin Immunother. 2018;14(1):140-145. doi: 10.1080/21645515.2017.1373921.
- Transgene receives FDA IND approval to begin a clinical trial with TG4010 + nivolumab + chemotherapy in the first-line treatment of lung cancer (NSCLC). Strasbourg, France: Transgene; September 11, 2017. transgene.fr/wp-content/ uploads/2017/09/20170911-PR-IND-TG4010-1L-EN.pdf. Accessed February 16, 2018.
- Beebe M, Qin M, Moi M, et al. Formulation and characterization of a ten-peptide single-vial vaccine, EP-2101, designed to induce cytotoxic T-lymphocyte responses for cancer immunotherapy. Hum Vaccin. 2008;4(3):210-218.
- Kotsakis A, Vetsika EK, Christou S, et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: results of an expanded phase II study. Ann Oncol. 2012;23(2):442-449. doi: 10.1093/annonc/mdr396.
- Gridelli C, Ciuleanu T, Domine Gomez M, et al. Randomized double blind phase IIb trial in advanced NSCLC patients who did not progress after first-line platinumbased chemotherapy: Vx-001, a therapeutic cancer vaccine vs placebo as maintenance therapy. Ann Oncol. 2017;28(suppl_5):v605-v649.
- Vaxon announces results of its phase IIb lung cancer trial of Vx-001, a therapeutic vaccine based on optimized cryptic peptides. Paris, France: Vaxon Biotech; June 1, 2017. vaxon-biotech.com/wp-content/uploads/2013/03/Vaxon-Biotech- 170601-Phase-2b-results-EN.pdf. Accessed February 16, 2018.
- Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217-221. doi: 10.1038/nature22991.
- Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222-226. doi: 10.1038/nature23003.
- Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29(1):84-91. doi: 10.1093/annonc/mdx755.
