Future Directions of Neoadjuvant Therapy in Muscle-Invasive Bladder Cancer
Cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy has been the standard of care in muscle-invasive bladder cancer for almost 2 decades. However, the rates of NAC utilization remain low and many patients are ineligible to receive cisplatin due to diminished renal function or other factors. Additionally, there are no reliable biomarkers routinely used in clinical practice that identify patients most likely to benefit from NAC, and limited prospective comparisons of the NAC regimens.
Vadim S. Koshkin, MD
ABSTRACT
Cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy has been the standard of care in muscle-invasive bladder cancer (MIBC) for almost 2 decades. However, the rates of NAC utilization remain low and many patients are ineligible to receive cisplatin due to diminished renal function or other factors. Additionally, there are no reliable biomarkers routinely used in clinical practice that identify patients most likely to benefit from NAC, and limited prospective comparisons of the NAC regimens. This paper begins by summarizing existing evidence to support the use of cisplatin-based neoadjuvant chemotherapy in patients with MIBC. It then discusses the likely challenges and issues that will shape neoadjuvant treatments for bladder cancer in the near future, and reviews emerging data regarding tumor biomarkers and molecular subtypes of MIBC that may help identify patients most likely to benefit from neoadjuvant treatment. The paper concludes by describing emerging novel neoadjuvant clinical trial designs in MIBC; such designs incorporate biomarker-driven and bladder-sparing approaches as well as novel immunotherapy regimens that include, but are not limited to, patients who are ineligible for cisplatin-based chemotherapy. The ongoing rapid expansion of knowledge about the underlying biology of bladder cancer and newly generated data regarding the efficacy of immunotherapy agents in this disease herald significant changes in the future direction of its neoadjuvant therapies.
Introduction
The established standard of care in muscle-invasive bladder cancer (MIBC) is cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy with bilateral pelvic lymph node dissection. There is well-established level I evidence for the benefit of NAC in this setting based on an overall survival (OS) advantage that was demonstrated in several randomized clinical trials. Unfortunately, many patients are ineligible for cisplatin-based therapy, most commonly due to diminished renal function but also as result of a number of other factors that may make a patient “unfit” for cisplatin. Consequently, many patients with MIBC have to forgo this potentially life-prolonging treatment. Given the established OS benefit of neoadjuvant systemic therapy with cisplatin-based combinations, there is considerable interest in identifying patients most likely to benefit from this approach and also in novel neoadjuvant therapies to address an unmet clinical need, including in cisplatin-ineligible patients. In particular, several trials of immunotherapy agents in the neoadjuvant setting are currently enrolling patients or will begin accrual in the coming months. For patients eligible to receive cisplatin, there are no prospectively validated molecular biomarkers to provide guidance on which patients are most likely to respond to treatment, although new data in genomics and molecular subtyping are beginning to shift this paradigm.
Evidence for Cisplatin-Based Neoadjuvant Chemotherapy
Cisplatin-based NAC has been the standard of care in MIBC for almost 2 decades, as established by evidence from several randomized clinical trials and a large meta-analysis. The meta-analysis, initially published by the Advanced Bladder Cancer Meta-analysis Collaboration in 20031 and updated in 2005,2included in its final version 11 randomized clinical trials and 3005 patients; it compared platinum-based NAC (1 trial included carboplatin, the rest cisplatin) plus definitive local therapy (cystectomy or radiation) with definitive local therapy alone. The results showed significant benefit for patients who received cisplatin-based NAC combination with a 14% reduction in risk of death (hazard ratio [HR], 0.86; 95% CI, 0.77-0.95; P = .003), which translated into a 5% improvement in OS and a 9% improvement in DFS at 5 years.2 The 5-year OS was 50% for patients who received cisplatin-based NAC compared with 45% for patients who received definitive local therapy alone. It is notable that a survival benefit was observed only for patients who received cisplatin-based chemotherapy combinations; the same benefit has not been shown for patients who received carboplatin-based combinations.
An illustrative trial included in this meta-analysis was SWOG 8710, which used MVAC (methotrexate, vinblastine, doxorubicin [Adriamycin], cisplatin) given every 28 days for a total of 3 cycles as the NAC regimen prior to radical cystectomy.3This trial included 317 patients enrolled from 1987 to 1998 with stage cT2-T4a MIBC who were intended to undergo radical cystectomy, and were randomized 1:1 to receive either 3 cycles of MVAC (28-day cycle) followed by radical cystectomy or radical cystectomy alone. Intention-to-treat analysis revealed median OS in the MVAC-and-cystectomy group to be 77 months compared with 46 months in the cystectomy group (P =.06). Patients who received MVAC had higher rates of pathologic complete response (pCR) at cystectomy (38% vs 15%; P <.001). In both groups, patients with pCR had improved survival, with 85% of patients with pCR remaining disease-free at 5 years.
Another large neoadjuvant study with long-term follow-up randomized 976 patients with high-grade cT2-T4a N0-NX MIBC to receive either CMV (cisplatin, methotrexate, vinblastine) chemotherapy for 3 cycles followed by local therapy (cystectomy or radiotherapy) or local therapy alone.4 Long-term follow-up revealed an OS advantage at the 10-year time point for patients receiving CMV (36% vs 30%) with a 16% reduction in the risk of death (HR, 0.84; 95% CI, 0.72-0.99; P = .037).
More recently, additional neoadjuvant regimens have been studied with the aim of improving patient tolerance and shortening treatment duration. These have included accelerated or dose-dense MVAC (ddMVAC) regimens administered in 2-week cycles with granulocyte colony stimulating factor (G-CSF) support (total of 3 to 4 cycles over 6 to 8 weeks), which have shown comparable pathologic response rates (pRRs) and a favorable profile of treatment-related adverse events compared with historical classic MVAC data.5-7In general, about 30% of patients achieved pCR and 40% to 50% had pathologic downstaging with ddMVAC. Another frequently used NAC regimen is the combination of gemcitabine and cisplatin (GC) administered in 21-day cycles for a total of 3 to 4 cycles (4 cycles corresponding to the 12-week duration of NAC in the SWOG 8710 trial). Retrospective comparisons of GC with MVAC in the neoadjuvant setting showed a similar rates of pCR in the 2 regimens, at around 30%.8,9 Dose-dense GC (ddGC) administered in 14-day cycles with G-CSF support (total of 3 cycles over 6 weeks) has also been investigated, with initial results showing pRRs comparable with MVAC and ddMVAC.10 A retrospective comparison of MVAC, GC, and ddMVAC has shown the regimens to be comparable in terms of pCR and partial (pPR) response rates, but with superior patient tolerance of the ddMVAC and GC regimens compared with classic MVAC.11
Currently, no approved biomarkers exist to assist in individualized treatment decisions of whether to pursue NAC for a particular patient, or to choose among the different NAC regimens. This may change following completion of the currently ongoing SWOG 1314 study, which randomizes MIBC patients to receive either ddMVAC or GC as NAC prior to cystectomy. The primary endpoint of this study is to prospectively validate the coexpression extrapolation (COXEN) score, which assesses tumor sensitivity to chemotherapy based on gene expression. This is a novel trial design in which the COXEN score generated based on gene expression in the transurethral resection of bladder tumor (TURBT) specimen will be assessed in its ability to predict the patient’s pathologic response at the time of cystectomy to either ddMVAC or GC NAC regimen.12Although not definitively powered to compare the efficacy of ddMVAC versus GC, this trial will provide insights by comparing the 2 regimens in a randomized prospective fashion.
The use of adjuvant chemotherapy following cystectomy in bladder cancer has also been investigated in several trials. The European Organisation for Research and Treatment of Cancer (EORTC) 30994 trial randomized patients with pT3-pT4 or N+ disease at cystectomy and no evidence of distant metastases to receive adjuvant chemotherapy with GC or MVAC within 90 days of treatment start or at the time of relapse. The trial was underpowered, enrolling only 284 of the intended 660 patients, and the 5-year OS had a trend favoring adjuvant chemotherapy but was not statistically different between the 2 groups. It did, however show an advantage, 48% versus 32% after 5 years, in progresson-free survival (PFS) for patients who received immediate adjuvant therapy (HR, 0.5; 95% CI, 0.40-0.73; P <.001).13 A meta-analysis of 9 randomized adjuvant trials that included 945 patients did show an OS advantage for patients receiving adjuvant therapy, with a pooled HR at 0.77 (95% CI, 0.59-0.99; P = .049) and a DFS advantage with HR at 0.66 (95% CI, 0.45-0.91; P = .014) and a more apparent DFS benefit in patients with nodal metastases.14 However, concerns have been raised regarding significant variability among individual trials included in the meta-analysis, such as variations in eligibility criteria, statistical designs, treatment in the control arm, and definitions of DFS. Benefit of adjuvant chemotherapy was also supported by a large retrospective study of more than 5600 patients with locally advanced bladder cancer (pT3-T4 or N+), in which adjuvant therapy was associated with improved OS in comparison with post cystectomy observation (HR, 0.70; 95% CI, 0.64-0.76; P <0.001).15 In general, although a degree of benefit with adjuvant treatment has been suggested, the data have been mixed and not as consistent as those data supporting cisplatin-based NAC. Right now, adjuvant cisplatin-based chemotherapy following radical cystectomy is generally considered for higher-risk patients (pT3-pT4 or N+) if they have not received prior cisplatin-based neoadjuvant therapy.
Molecular Subtypes, Mutations, and Other Putative Predictive Biomarkers
Current treatment recommendations for cisplatin-based NAC in MIBC follow a “one size fits all” approach, but new data on predictive biomarkers are emerging. Presence of genomic alterations in genes such as ERBB2, ERCC2, and RB1, as well as other genes involved in DNA-repair pathways (eg, ATM, FANCC), has been shown to be potentially predictive of response to cisplatin-based NAC.16-20Additionally, the recent discovery of several distinct molecular subtypes in urothelial carcinoma has considerably advanced our understanding of the underlying biology of this disease, yielding information that may impact treatment decisions in the near future. The results of 4 distinct molecular classification methods have been published,18,21-23all of which broadly divide urothelial carcinomas into basal and luminal tumors and then into more narrow subtypes specific to each method. The most comprehensive integrative molecular analysis was undertaken by The Cancer Genome Atlas (TCGA), initially on 131 tumors in 201421 and updated with 412 samples in 2017.24,25The updated analysis identified 5 distinct molecular subtypes that were prognostic of outcomes and suggested potential treatment approaches with either cisplatin-based chemotherapy, immunotherapy, or targeted agents. An important recent study published by Seiler and colleagues was the first to use a single-sample classifier (Decipher assay) to retrospectively generate molecular subtypes based on whole transcriptome profiling of TURBTs in 343 patients with MIBC.26This genomic subtyping classifier was able to accurately predict the previously described consensus molecular subtypes and suggested improved clinical outcomes in patients with luminal tumors. Exploratory analysis additionally suggested that tumors with the basal subtype derived the most benefit from NAC. Following publication of this study, GenomeDx Biosciences launched Decipher Bladder Cancer Classifier, the first commercially available clinical assay to subtype individual MIBC samples.
These are all preliminary findings that need to be validated prospectively. However, the evidence for the eventual individualization of treatment patterns in MIBC based on tumor genomics and molecular subtyping is growing. In the near future, these emerging diagnostic tools may be utilized to help make difficult treatment decisions about prioritizing certain MIBC patients for cisplatin-based NAC and others for targeted agents and/or immunotherapy. This will both help improve outcomes in this patient population and help avoid cisplatin-related toxicity in patients least likely to benefit from this treatment approach. It may additionally suggest potential treatment options in patients with MIBC who are cisplatin-unfit and who currently lack proven systemic treatment options.
Cisplatin Ineligibility and Low Rates of NAC Utilization
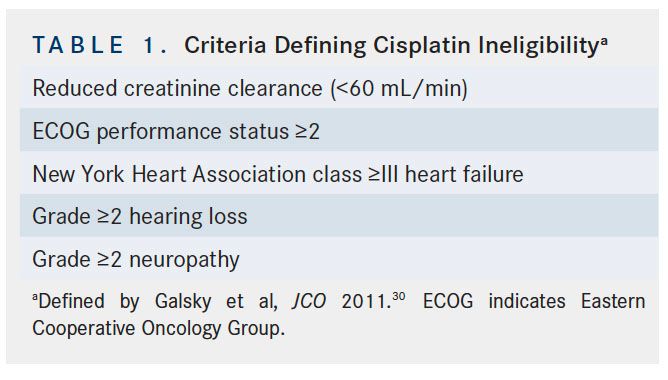
Despite the proven OS benefit of cisplatin-based NAC prior to cystectomy, NAC utilization rates for patients with MIBC remain low and are thought to be under 20%.27,28The main concerns raised by healthcare providers about recommending NAC use for patients with MIBC include questions regarding treatment toxicity and potential delay of curative radical cystectomy.29Many patients with MIBC are unfortunately ineligible for cisplatin-based chemotherapy. Currently established criteria for cisplatin ineligibility are derived from a survey of genitourinary medical oncologists and include any of the following: ECOG performance status ≥2, creatinine clearance <60 mL/min, grade ≥2 neuropathy, grade ≥2 hearing loss, and/or New York Heart Association class ≥III heart failure (Table 1).30 These guidelines are not based on prospective evidence and efforts are underway to better define specific eligibility thresholds for treatment with cisplatin, especially for patients with diminished renal function. Improvement in definitions of cisplatin eligibility and continued education of providers, especially in community settings, can go a long way toward increasing utilization rates of this potentially life-prolonging treatment.
Past, Current, and Future Trials of Novel Agents in the Neoadjuvant Setting
The desire to enhance the efficacy and outcomes with cisplatin-based NAC while mitigating the toxicities of this treatment has led to the consideration of alternative combinations that include targeted agents and immune checkpoint inhibitors. A regimen of gemcitabine and cisplatin in combination with sunitinib (VEGFRtyrosine kinase inhibitor) was investigated in phase II trials in both advanced and neoadjuvant settings, but both trials had to be terminated due to excessive toxicities even after dose reductions. In the neoadjuvant trial, 2 of 9 patients achieved pCR at cystectomy.31A small phase II trial of neoadjuvant cisplatin, gemcitabine, and bevacizumab (anti-VEGF antibody) produced downstaging in 4 of 13 patients with MIBC, but the regimen was hampered by postoperative complications observed in 5 of 12 patients.32Another phase II trial used erlotinib (an EGFR tyrosine kinase inhibitor) monotherapy as neoadjuvant treatment in 20 patients with MIBC and resulted in pCR in 5 (25%) and pPR in 7 (35%) patients.33An ongoing trial in the United Kingdom is using nintedanib (FGFR and VEGFR inhibitor) with gemcitabine and cisplatin versus gemcitabine and cisplatin as neoadjuvant therapy for MIBC (Cancer Research United Kingdom internal database number 11977).34
The efficacy of immune checkpoint inhibitors (ICIs), including antiPD-1 and anti–PD-L1 agents, in advanced urothelial carcinoma has encouraged their use in clinical trials in the neoadjuvant setting, particularly in light of the better tolerability of these agents compared with cytotoxic chemotherapy or targeted agents. A number of neoadjuvant trials have also considered bladder-sparing approaches, especially in patients whose tumors harbor a relevant genomic profile that may render them more susceptible to systemic therapies. Hoosier Cancer Research Network’s Genitourinary 16-257 trial is a study of neoadjuvant gemcitabine, cisplatin, and nivolumab in MIBC patients that includes selective biomarker-based bladder sparing. This phase II trial builds on the concept that patients with urothelial cancers harboring DNA-damage response (DDR) mutations might be particularly sensitive to the combination of immune checkpoint (IC) blockade plus cisplatin-based chemotherapy. Prior data have independently demonstrated that DDR alterations confer sensitivity to cisplatin-based chemotherapy and to IC blockade.16,35,36In this study, patients with MIBC will receive 4 cycles of gemcitabine and cisplatin plus nivolumab after having targeted exome sequencing of their baseline TURBT specimen. Patients who achieve clinical CR and have tumors with 1 of 5 DDR-related biomarkers will be offered maintenance nivolumab without cystectomy, while the remaining patients will undergo standard cystectomy. The primary endpoint of this trial, which is not yet open, will be metastasis-free survival. Another similar phase II study of a novel organ-sparing approach sponsored through the Alliance for Clinical Trials in Oncology (A031701) will be testing the ability to use deleterious DDR gene alterations to help identify patients most likely to achieve pathologic downstaging of their disease following cisplatin-based
chemotherapy, who could be managed with surveillance alone without radical cystectomy. Another trial (NCT02710734) is evaluating a risk-adapted approach to MIBC therapy. Each baseline TURBT sample will be sequenced while proceeding with neoadjuvant accelerated (A) MVAC chemotherapy. Based on the mutational profile and the post-AMVAC TURBT findings, patients will be treated with active surveillance (experimental arm), or standard of care intravesical therapy, chemoradiation, or surgery.
For patients who are ineligible for cisplatin-based NAC according to expert consensus guidelines, the current standard of care is to proceed directly to radical cystectomy with bilateral pelvic lymph node dissection or to enroll into a clinical trial. Many patients get carboplatin-based regimens in this setting; however, as was stressed previously, there is no high-level evidence supporting this approach and carboplatin-based regimens are not recommended in the neoadjuvant or adjuvant setting outside clinical trials. Given significant rates of recurrence in this patient population, estimated at about 50%, current efforts are investigating alternative systemic treatment options. Given the superior adverse effect profile and much better tolerability of ICIs compared with conventional cytotoxic chemotherapy, their potential use in cisplatin-ineligible patients is particularly intriguing. Several currently ongoing trials in this treatment space are of interest.
Atezolizumab, an antiPD-L1 agent, is currently approved in metastatic urothelial cancer37and is now being studied in the neoadjuvant setting. A single-arm, phase II study of atezolizumab administered as neoadjuvant therapy to patients with muscle-invasive urothelial carcinoma who are appropriate for cystectomy but are ineligible for or refuse NAC is currently accruing patients. The primary endpoints of this trial are change in CD3+ T-cell density and rate of pCR at cystectomy (NCT02451423).38Another trial is assessing the use of pembrolizumab, an antiPD-1 agent and current standard of care in platinum-refractory metastatic urothelial cancer,39as neoadjuvant therapy. This phase Ib/II study is using the combination of gemcitabine/cisplatin and pembrolizumab in cisplatin-eligible patients, or gemcitabine and pembrolizumab in cisplatin-ineligible patients, as neoadjuvant therapy followed by radical cystectomy. The primary endpoint in this trial is rate of pathologic response at cystectomy, and the study will likely complete accrual in 2018 (NCT02365766).40
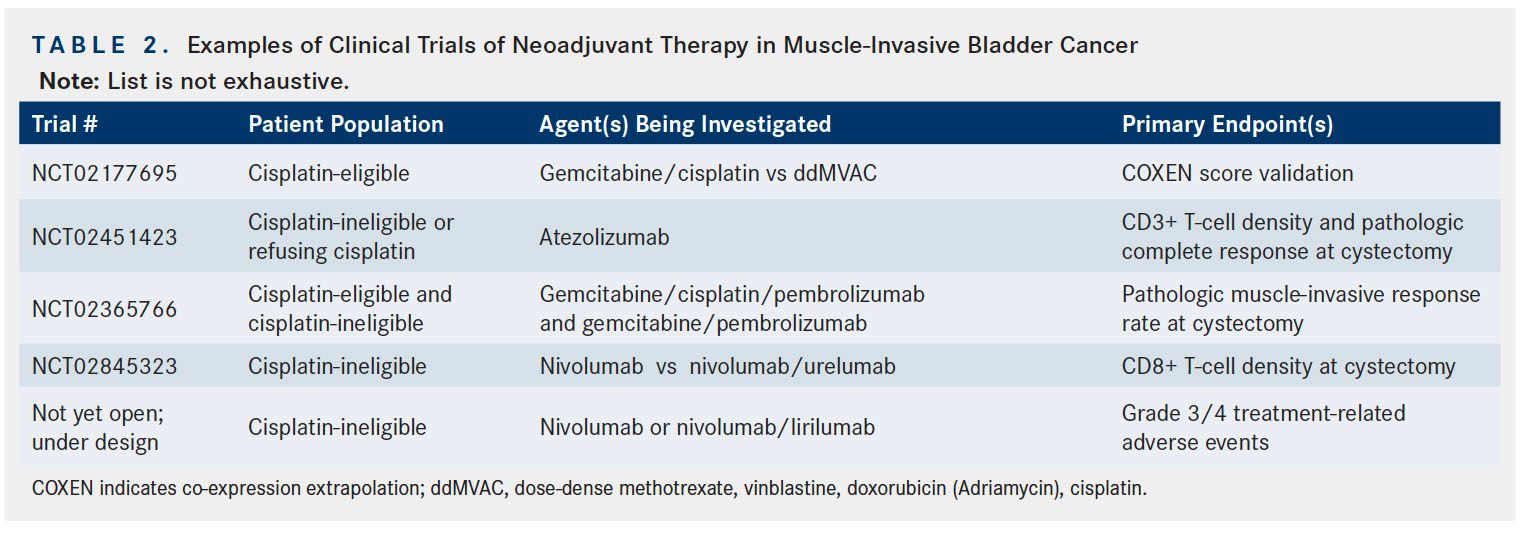
Nivolumab is another antiPD-1 agent approved for the treatment of metastatic urothelial cancer refractory to platinum-based therapy and whose potential role as neoadjuvant therapy in bladder cancer is currently under investigation.41A recently opened 2-arm phase II trial is assessing nivolumab monotherapy and nivolumab in combination with urelumab (anti-CD137 agent) as neoadjuvant therapy prior to radical cystectomy in cisplatin-ineligible patients with MIBC. The primary endpoint in this trial is CD8+ T-cell density at cystectomy, with pathologic response at cystectomy as 1 of the secondary endpoints (NCT02845323).42A similarly structured phase Ib trial of patients treated with nivolumab monotherapy or combination of nivolumab and lirilumab (anti-killer cell immunoglobulin-like receptor) antibody that upregulates activity of NK cells) who are cisplatin-ineligible, will likely begin accruing patients in the coming months via PrECOG. Additional neoadjuvant trials of nivolumab and other agents are currently being designed. A list of representative neoadjuvant trials in bladder cancer that are currently accruing patients or will soon be opening to accrual is provided in Table 2.
Conclusions
After a long period of stagnation, the last several years have seen dramatic changes in the treatment of advanced bladder cancer. Although these have not yet altered standard practice in the administration of neoadjuvant treatment for MIBC, such changes are on the horizon. In the treatment of patients eligible for the current standard of care with cisplatin-based chemotherapy combinations, the field is gradually moving away from a “one size fits all” approach into more individualized treatment decisions driven by novel biomarkers, pending further validation. For patients who are cisplatin-ineligible, novel agents and combinations are being investigated in clinical trials. Our increasing understanding of the underlying tumor biology and immunology in this aggressive disease is driving the evaluation of new agents in the neoadjuvant setting for MIBC.
References:
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361(9373):1927-1934.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) metaanalysis collaboration. Eur Urol. 2005;48(2):202-205; discussion 205-206.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer [published correction appears in N Engl J Med. 2003;349(19):1880]. N Engl J Med. 2003;349(9):859-866.
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171-2177. doi: 10.1200/JCO.2010.32.3139.
- Blick C, Hall P, Pwint T, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC) as neoadjuvant chemotherapy for patients with muscleinvasive transitional cell carcinoma of the bladder. Cancer. 2012;118(16):3920- 3927. doi: 10.1002/cncr.26675.
- Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscleinvasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32(18):1889-1894. doi: 10.1200/JCO.2013.52.4785.
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32(18):1895-1901. doi: 10.1200/JCO.2013.53.2465.
- Dash A, Pettus JA 4th, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113(9):2471-2477. doi: 10.1002/cncr.23848.
- Galsky MD, Pal SK, Chowdhury S, et al; Retrospective International Study of Cancers of the Urothelial Tract (RISC) Investigators. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121(15):2586-2593. doi: 10.1002/cncr.29387.
- Plimack ER, Hoffman-Censits JH, Kutikov A, et al. Neoadjuvant dose-dense gemcitabine and cisplatin (DDGC) in patients (pts) with muscle-invasive bladder cancer (MIBC): final results of a multicenter phase II study. J Clin Oncol. 2014;32(suppl 5s; abstr 4513).
- van de Putte EE, Mertens LS, Meijer RP, et al. Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J Urol. 2016;34(2):157-162. doi: 10.1007/s00345-015-1636-y.
- S1314, co-expression extrapolation (COXEN) program to predict chemotherapy response in patients with bladder cancer. ClinicalTrials.gov website. clinicaltrials. gov/ct2/show/NCT02177695. Updated July 13, 2017. Accessed August 26, 2017.
- Sternberg CN, Skoneczna I, Kerst JM, et al; European Organisation for Research and Treatment of Cancer Genito-Urinary Cancers Group; Groupe d’Etude des Tumeurs Urogénitales; National Cancer Research Institute Bladder Cancer Study Group; et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(1):76-86. doi: 10.1016/S1470-2045(14)71160-X.
- Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66(1):42-54. doi: 10.1016/j.eururo.2013.08.033.
- Galsky MD, Stensland KD, Moshier E, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34(8):825- 832. doi: 10.1200/JCO.2015.64.1076.
- Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol. 2015;68(6):959-967. doi: 10.1016/j.eururo.2015.07.009.
- Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol. 2016;2(8):1094-1096. doi: 10.1001/jamaoncol.2016.1056.
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152-165. doi: 10.1016/j.ccr.2014.01.009.
- Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. 2016;69(3):384-388. doi: 10.1016/j.eururo.2015.01.014.
- Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4(10):1140-1153. doi: 10.1158/2159-8290.CD-14-0623.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315- 322. doi: 10.1038/nature12965.
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111(8):3110-3115. doi: 10.1073/pnas.1318376111.
- Sjödahl G, Lauss M, Lövgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18(12):3377-3386. doi: 10.1158/1078-0432.CCR-12-0077-T.
- Lerner SP, Robertson G, Kim J, et al. Comprehensive molecular characterization and analysis of muscle-invasive urothelial carcinomas. J Clin Oncol. 2017;35(15 suppl; abstr 4500). doi: 10.1200/JCO.2017.35.15_suppl.4500.
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540-556. e25. doi: 10.1016/j.cell.2017.09.007.
- Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscleinvasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017;72(4):544-554. doi: 10.1016/j.eururo.2017.03.030.
- Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117(2):276-282. doi: 10.1002/cncr.25429.
- Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185(1):72-78. doi: 10.1016/j.juro.2010.09.015.
- Cowan NG, Chen Y, Downs TM, et al. Neoadjuvant chemotherapy use in bladder cancer: a survey of current practice and opinions. Adv Urol. 2014;2014:746298. doi: 10.1155/2014/746298.
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432-2438. doi: 10.1200/JCO.2011.34.8433.
- Galsky MD, Hahn NM, Powles T, et al. Gemcitabine, cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2013;11(2):175-81. doi: 10.1016/j.clgc.2012.10.001.
- Chaudhary UB, Golshayan AR, Brisendine A, et al. Phase II trial of neoadjuvant cisplatin, gemcitabine, and bevacizumab followed by radical cystectomy (RC) in patients with muscle-invasive transitional cell carcinoma (TCC) of the bladder. J Clin Oncol. 2011;29(suppl_7; abstr 276). doi: 10.1200/jco.2011.29.7_suppl.276.
- Pruthi RS, Nielsen M, Heathcote S, et al. A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: clinical and pathological results. BJU Int. 2010;106(3):349-354. doi: 10.1111/j.1464-410X.2009.09101.x.
- Aragon-Ching JB, Trump DL. Targeted therapies in the treatment of urothelial cancers. Urol Oncol. 2017;35(7):465-472. doi: 10.1016/j.urolonc.2017.03.011. 35. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733.
- Teo MY, Seier K, Ostrovnaya I, et al. DNA damage repair and response (DDR) gene alterations (alt) and response to PD1/PDL1 blockade in platinum-treated metastatic urothelial carcinoma (mUC). J Clin Oncol. 2017;35(suppl_15; abstr 4509). doi: 10.1200/JCO.2017.35.15_suppl.4509.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. doi: 10.1016/S0140-6736(16)00561-4.
- Study of MPDL3280A in bladder cancer. ClinicalTrials.gov website. clinicaltrials. gov/ct2/show/NCT02451423. Updated June 8, 2017. Accessed August 29, 2017. 39. Bellmunt J, de Wit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683.
- Neoadjuvant pembrolizumab in combination with gemcitabine therapy in cis-eligible/ ineligible UC Subjects. clinicaltrials.gov website. clinicaltrials.gov/ct2/show/study/ NCT02365766. Updated December 22, 2017. Accessed January 27, 2018.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7.
- Neoadjuvant nivolumab with and without urelumab in patients with cisplatinineligible muscle-invasive urothelial carcinoma of the bladder. ClinicalTrials.gov website. clinicaltrials.gov/ct2/show/NCT02845323. Updated August 1, 2017. Accessed August 29, 2017.