Preclinical Findings Illuminate Intricacies in CDK4&6 Inhibition
Inhibition of cyclin-dependent kinase 4 (CDK4) and CDK6 has been extensively studied in estrogen receptor (ER)‒driven breast cancer.
CDK4 and CDK6-related oncogenesis is not limited to breast cancer. Recent data from preclinical trials reveal that the cyclin D:CDK4 and CDK6 complex drives dysregulated cell proliferation in other cancer types. In addition, these preclinical findings shed light on the interactions between CDK4 and CDK6 and other oncogenes, which may hold relevance as potential biomarkers and provide new marks for targeted therapies.
The Role of CDK4 Expression in Cancer
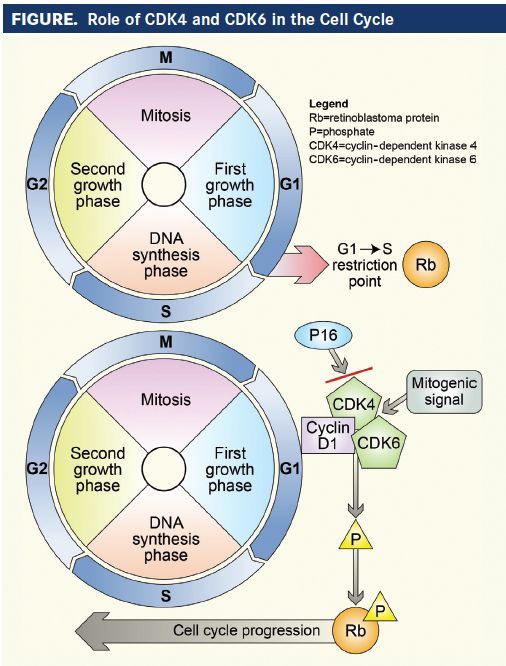
CDK4 belongs to the family of serine and threonine protein kinases, and is involved in regulating the checkpoint between the G1 and S phases of the cell cycle.1When D-type cyclins are activated by mitogenic signals, they bind to and activate CDK4 and CDK6, which have similar amino acid makeup and functions.2,3CDK4 has a high and nearly exclusive specificity for the retinoblastoma tumor suppressor gene product (pRb).2By phosphorylating pRb, CDK4 allows the release of previously sequestered E2F transcription factors, which then drive the transition from G1 to S phase. E2F transcription factors also phosphorylate pRb, leading to further release of E2F, creating a positive feedback loop. As a result, upregulated CDK4 activity induces cells to undergo an irreversible transition to the S phase, and to continue propagating independent of mitogen signals.1
The activity of the cyclin D:CDK4 and CDK6 axis is inhibited, however, by p16INK4a and the CIP/KIP family of proteins (p21, p27, p56). When the cell encounters inhibitory signals, such as transforming growth factor-β, p16INK4a and CIP/KIP protein synthesis rapidly increases. These negative regulatory molecules then bind to and inhibit the cyclin D:CDK4 and CDK6 complex. Functional p16INK4a appears to be necessary to activate cellular senescence, and loss of p16INK4a is involved in the pathogenesis of many cancers.1
While dysregulation of the cyclin D:CDK4 and CDK6 axis mediates tumorigenesis in several types of cancer, the role of CDK4 in oncogenesis has been most comprehensively studied in breast cancer. Preclinical studies have shown that the ER and HER2 signaling pathways both exert downstream effects on the cyclin D:CDK4 and CDK6 process. Because 60% of breast cancers are ER-positive and 25% are HER2-positive, the cyclin D:CDK4 and CDK6 complex is central to tumorigenesis for the majority of breast cancers.1
CDK4 also plays an important role in developing resistance to therapy for both ER-positive and HER2- positive breast cancers. In HER2-positive tumors, cyclin D1:CDK4 mediates resistance to hormonal treatment. In HER2-positive mouse models and human breast cancer cell lines, CDK4&6 inhibitors have been shown to resensitize tumor cells to anti-HER2 treatment. CDK4&6 inhibitors attenuated mTORC1 activity by decreasing TSC2 phosphorylation, thereby reducing feedback inhibition of upstream epidermal growth factor receptor (EGFR) kinases and restoring tumor sensitivity to anti- EGFR/HER2 agents.4
In ER-positive breast cancer, ER signaling upregulates CDK4 and CDK6 levels by increasing cyclin D1 levels and enhancing other signaling pathways. The link between D-type cyclins and breast cancer is further illuminated by the finding that most breast cancers with upregulated cyclin D1 are ER-positive. Acquired resistance to hormone therapy may occur as a result of ER-independent dysregulation of mitogenic pathways that potentiate the cyclin D1:CDK4 and CDK6 axis.1
Increased CDK4 activity is essential for oncogenesis in other cancer types, as well. In melanoma, CDK4&6 inhibition destabilized FOXM1 and induced senescence in mouse models and human cell lines, suggesting that FOXM1 acts as an alternate substrate for CDK4 and CDK6. Nonsmall cell lung cancer (NSCLC) models with KRAS-driven disease demonstrated apoptosis after CDK4 inhibition. This effect was sustained in the clinical setting: A subsequent phase I study of CDK4&6 inhibitors in NSCLC showed that patients with the KRAS mutation had higher disease control rates (DCRs) than those who did not.2
In liposarcoma cell lines with chromosome 12q14 amplification of linked CDK4 and MDM2, CDK4&6 inhibitor palbociclib induced G1 cell cycle arrest within 48 hours. After palbociclib was removed, only the cells that expressed senescence biomarkers remained quiescent. In addition, proteolytic turnover of MDM2 was necessary to maintain senescence. Among patients from whom the cell lines were acquired, those with significantly reduced MDM2 expression had the greatest clinical benefit, with progression-free survival (PFS) ranging from 160 days to more than 800 days. Of note, pRb phosphorylation was reduced in clinical responders, as well.2
Finally, preclinical models of glioblastoma multiforme found that the presence of simultaneous deletions in the genes that encode p16INK4a and p18INK4c (CDKN2A and CDKN2C, respectively) was associated with increased sensitivity to CDK4&6 inhibitors.2
The wealth of new information from preclinical studies indicates that CDK4 plays a significant role in driving oncogenesis in many cancer types outside of breast cancer. Further elucidating the mechanisms of CDK4-induced oncogenesis in these cancers will provide the key to developing more-effective targeted therapies, and clinical trials will help determine the potential of CDK4&6 inhibitors in treating malignancies other than breast cancer.
FIGURE. Role of CDK4 and CDK6 in the Cell Cycle
The Connection Between CDK6 and DLC1
A preclinical study by Xiaofeng Dai, with the School of Biotechnology and National Engineering Laboratory for Cereal Fermentation Technology at Jiangnan University in Wuxi, China, and colleagues demonstrated the link between deleted in liver cancer- 1 (DLC1) and CDK6. DLC1 is a tumor suppressor gene that is inactivated by deletions or DNA methylation in many cancers. When functioning normally, DLC1 deactivates RhoGTP by catalyzing its conversion to RhoGDP. As a Rho family GTPase, DLC1 acts as a molecular switch that promotes tumorigenesis via cell cycle progression and malignant transformation. Preclinical data shows that reactivating DLC1 suppresses tumor cell proliferation.3
Synergy between DLC1 and CDK6 has been demonstrated in lung, colon, and breast cancers. The combination of high DLC1 and low CDK6 expression correlates with a good prognosis, and the converse denotes poor prognosis. When the DLC1 intronic single nucleotide polymorphism (SNP) rs561681 is heterozygous and occurs with the rare homozygous variant of CDK6 intronic SNP rs3731343, the effect is deleterious; however, when heterozygous rs561681 occurs with the common homozygote of SNP, rs3731343, the effect is protective. The link between DLC1 and CDK6 is also demonstrated by the function of caveolin 1, which enhances the tumor suppressive function of DLC1 while inhibiting CDK6 via downstream effects.3
Results from this study also demonstrated a connection between ER, DLC1, and CDK6. In ER-positive breast cancer models, DLC1 enabled anchorage-independent cell growth via estrogen-induced transactivation of liganded ER. Estrogen also induced DLC1 transcription and expression. D-type cyclins may act upstream of the ER pathway to potentiate estrogeninduced transactivation.3
In ER-positive breast cancer cells, nonrare homozygotes of DLC1 SNP rs561681 have a protective effect, which is enhanced by the presence of nonrare CDK6 SNP rs3731343 homozygotes. As a result, outcomes tend to be better in patients without mutations, with high DLC1 expression and no CDK6 overexpression. On the other hand, rare homozygotes of DLC1 SNP rs561681 have low copy numbers of DLC1, which correlates with a poor outcome. Finally, heterozygous dimers of DLC1 SNP rs561681 exert a dominant negative effect on CDK6 SNP rs3731343, in which levels of functional DLC1 are lowest in cells with heterozygous DLC1 SNP rs561681 and rare homozygous CDK6 SNP rs3731343.3
The authors concluded that, “In this study, we have linked the germline genetic polymorphisms and their synergistic effect with breast cancer progression, which provides the basis for experimentally elucidating the mechanisms involved in differential progression. The findings are particularly useful in investigating inherited susceptibility and their cooperative roles in tumor progression, with the ultimate goal of tailoring clinical treatments for breast cancer patients based on their genetic susceptibility.”3
Continuous Inhibition and Cell Senescence
Continuous inhibition of the cyclin D:CDK4 and CDK6 axis appears to be required to maintain cellular senescence. In mouse models, senescence may be reversed by inactivating pRb through phosphorylation. The presence of functional pRband by corollary, inhibition of the cyclin D:CDK4 and CDK6 axis—appears to be necessary to induce and maintain senescence.5
Sara Tolaney, MD, with Dana-Farber Cancer Institute in Boston, MA, noted that, “Preclinical data suggests that continuous administration of CDK4&6 inhibitors may allow for continued cellular senescence.” In xenograft cancer models, tumors resumed growth after palbociclib was withdrawn, but reintroducing palbociclib halted further tumor progression. In mouse models, pharmacokinetic and pharmacodynamic studies showed that dosing abemaciclib at a frequency of twice daily was necessary to ensure adequate target coverage.2
The importance of continuous dosing plays out in the clinical setting as well. Patients with melanoma who were treated with abemaciclib once daily had evidence of partial reversibility in skin biopsies before the next dose. Even when each individual dose of abemaciclib was lowered, maintaining a continuous dosing regimen appeared to be sufficient for achieving target coverage. In phase I and II trials of abemaciclib for breast cancer treatment, pharmacodynamic CDK4&6 inhibition was maintained even when the dosage was decreased from 200 to 150 mg twice daily for side effects.2
Unlike palbociclib and ribociclib, which must be dosed on a schedule with a week off each cycle, abemaciclib does not often result in significant neutropenia, and its continuous dosing regimen is well-tolerated.2“Being able to administer abemaciclib on a continuous basis may explain its higher response rates as a single agent, compared with palbociclib and ribociclib,” Tolaney stated.
Combination Therapy and Treatment Sequencing
The mechanism of action of CDK4&6 inhibitors may present a problem when they are combined with other anticancer agents. “Because CDK4&6 inhibitors cause cell cycle arrest, there have been concerns that they may induce senescence in T-cells and therefore dampen the immune system,” Tolaney acknowledged. Immune checkpoint inhibitors used as anticancer agents are antibodies that bind to negative regulatory molecules, such as programmed cell death 1 (PD-1)/programmed death ligand 1 (PDL1), in order to induce T-cell proliferation and differentiation. But T-cell expansion is dependent on the cyclin D:CDK4 and CDK6 axis and may be constrained by CDK4&6 inhibition.2
Tolaney indicated that CDK4&6 inhibitors may not blunt the effects of immune checkpoint inhibitors. She noted that “preclinical data looking at T-cell populations before and after exposure to CDK4&6 inhibitors suggest that the abundance of Tcells is largely preserved with a reduction in regulatory T-cells. Expression of immune checkpoint proteins on CD8 cells was also reduced.” These findings provided the rationale for combining abemaciclib with pembrolizumab as a treatment strategy for breast cancer in a phase I trial (NCT02079636).
Another concern is that CDK4&6 inhibitors may counteract the effects of certain cytotoxic cancer treatments by inducing cell cycle arrest in the G1 phase in both cancerous and healthy cells.2As a result, cancer cells may escape apoptosis from ionizing irradiation and DNA-damaging agents, which target cells in the S or M phases. However, sequencing treatments may help circumvent this potential problem.
In addition, CDK4&6 inhibitors may be used to protect normal hematopoietic cells during cytotoxic chemotherapy. For example, palbociclib during carboplatin administration decreased rates of myelosuppression in a Rb-deficient, HER2-driven breast cancer mouse model. Thus, using CDK4&6 inhibitors in Rb-negative tumors may attenuate dose-limiting hematologic toxicities that occur with chemotherapy.2A phase I/II trial in Rb-negative NSCLC is currently studying the potential benefits of combining G1T28, a CDK4&6 inhibitor, with carboplatin and etoposide (NCT02499770).
Whether using CDK4&6 inhibitors with cytotoxic cancer treatments is problematic remains to be determined. Further studies are needed to establish optimal treatment schedules for drugs that may antagonize each other’s effects, and to explore the potential hemo-protective effects of combination treatment.2
REFERENCES
- Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers.Breast Cancer Res. 2016;18(1):17.
- Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy.Cancer Discov.2016;6(4):353-367.
- Dai X, Li L, Liu X, Hu W, et al. Cooperation of DLC1 and CDK6 affects breast cancer clinical outcome. G3 (Bethesda). 2014;5(1):81-91.
- Goel S, Wang Q, Watt AC, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors.Cancer Cell.2016;29(3):255-269.
- Takahashi A, Ohtani N, Hara E. Irreversibility of cellular senescence: dual roles of p16INK4a/Rb-pathway in cell cycle control.Cell Div. 2007;2:10.
Recent preclinical findings have revealed that the activity of CDK4 and CDK6 is interwoven with multiple pathways that drive oncogenesis in many cancers besides breast cancer. In addition, a link between DLC1 and CDK6 has been discovered that predicts breast cancer progression, and may one day provide prognostic information for lung and colon cancers, as well. Preclinical data illustrating the necessity of continuous dosing in CDK4&6 inhibition will likely guide the development of future agents. Understanding the interdependence and synergy between the cyclin D:CDK4 and CDK6 axis and other oncogenic pathways is central to developing effective treatment regimens, identifying potential therapy-related problems, and discovering novel combinations that may enhance existing strategies.
HER2-Low and -Ultralow Populations Benefit from T-DXd in HR+ mBC
November 13th 2024During a Case-Based Roundtable® event, Aditya Bardia, MD, MS, FASCO, discussed data from the DESTINY-Breast04 and DESTINY-Breast06 trials for HER2-low breast cancer in the second article of a 2-part series.
Read More