Mohamed Discusses Newer Chemoimmunotherapy Regimens in Squamous NSCLC
During a Targeted Oncology™ Case-Based Roundtable™ event, Mohamed K. Mohamed, MD, PhD, and participants discussed cemiplimab plus chemotherapy and tremelimumab/durvalumab plus chemotherapy as regimens for a patient with metastatic squamous non–small cell lung cancer.
Mohamed K. Mohamed, MD, PhD (MODERATOR)
Director of Thoracic Oncology
Cone Health Cancer Center
Greensboro, NC

EVENT REGION Mid-Atlantic and South Atlantic
PARTICIPANT LIST Daya Sharma, MD | Arun Bhandari, MD | Ayodele Ayoola, MD | Anusha Madadi, MD | Kenneth Hoffman, MD | Gabor Varadi, MD | Kwabena Osei-Boateng, MD | Harish Madala, MD
CASE SUMMARY
A man aged 67 years presented with a lump in the left deltoid muscle but no other signs or symptoms. He had a medical history of hypertension and osteoarthritis and is a former smoker with 5 pack-years. Ultrasound confirmed a 5-cm soft tissue mass in the left deltoid. Biopsy showed thyroid transcription factor 1–negative squamous cell carcinoma.
CT scan of the chest, abdomen, and pelvis showed the intramuscular mass in left deltoid; subcutaneous deposits in the back and thigh; a 5-cm right lower lobe spiculated mass; bilateral, bulky mediastinal lymphadenopathy; and a 4-cm right adrenal mass. Brain MRI showed no evidence of central nervous system metastasis. Staging was T2aN3M1b.
DISCUSSION QUESTION
- What newer chemoimmunotherapy regimens have been approved for patients with non–small cell lung cancer (NSCLC), and for which patients are they appropriate?
MOHAMED: Cemiplimab [Libtayo] plus platinum-based chemotherapy was approved November 8, 2022.1 The approval includes first-line adult patients with [NSCLC] with no EGFR, ALK, or ROS1 alteration, and they include patients with metastatic disease and also patients with locally advanced disease who were not candidates for surgical resection or definitive chemoradiation, so that’s an option for patients with earlier-stage disease….
On the other hand, tremelimumab [Imjudo] with durvalumab [Imfinzi] plus platinum-based chemotherapy was approved November 10, 2022, and it’s for patients with metastatic NSCLC with no sensitizing EGFR or ALK [genomic aberrations].2
SHARMA: Where exactly do you use durvalumab plus tremelimumab? Would you use it in patients who have mutations like STK11 and KRAS, or in all patients?
MOHAMED: The approval is in all patients, but I understand your point regarding the subset analysis that showed some effect on the KRAS and STK11 [population].3 These are exploratory subset analyses, but it’s something that when you see some patients with KRAS and SDK11, you may have to think about [whether] that is the right option for this patient. But the approval is for everyone, squamous and nonsquamous.
BHANDARI: It says EGFR, ALK, and ROS1 mutations [ for cemiplimab]. What if they have other mutations? [And] why do tremelimumab and durvalumab only [exclude] ALK and EGFR, not other mutations?
MOHAMED: It’s a good question. It’s how the trials were designed. When it comes to the practice, all of us would look at the mutation status and decide which patients would benefit from first-line [targeted therapy] rather than chemoimmunotherapy.
BHANDARI: [If they have another] mutation, will you not use those targeted therapies? Because if you go by the indication, it’s only [patients with mutated] EGFR, ALK, or ROS1 [who should not receive frontline chemoimmunotherapy].
MOHAMED: Yes, that’s true because most of the clinical trials starting from the time of pembrolizumab [Keytruda] have been excluding only EGFR and ALK, but when it comes to practice, I personally prefer targeted therapy first before consideration of immunotherapy and chemotherapy. If you look at the National Comprehensive Cancer Network [NCCN] guidelines for the patients with mutations, they recommend for you to use targeted therapy first for the patients who have actionable mutations approved in the first-line setting, but this is how the trials were designed.4
BHANDARI: Thank you.
MOHAMED: Have you had the opportunity to use cemiplimab for lung cancer either as a monotherapy or in combination with chemotherapy? Do you have any experience with cemiplimab in other types of cancer?
SHARMA: Yes, I use it in skin cancer all the time.
MOHAMED: What’s your experience with it?
SHARMA: [The patients] responded very well.
MOHAMED: For those who use it for lung cancer, can anyone tell us about their experience using it either as monotherapy or in combination?
BHANDARI: It worked very well.
AYOOLA: I’ve used it as monotherapy in an [older] woman with PD-L1 of about 80%. So far, she’s doing well. She’s tolerating it well. I got comfortable with it when I used it in skin cancer.
MOHAMED: It looks [like] most of the physicians who started using it in lung cancer are the ones who have experience with it in skin cancer and that got translated to lung cancer later…. Have you had the opportunity to use durvalumab plus tremelimumab for advanced lung cancer?
MADADI: I’ve used it in a patient who [had] refused chemotherapy and found it durable. Their PD-L1 was [approximately] 50%.
MOHAMED: Durvalumab and tremelimumab [are used with] 4 cycles of chemotherapy with this combination. The advantage of it is that the tremelimumab, the CTLA-4 inhibitor, is used for only 5 cycles—the 4 initial cycles and then 1 more at week 16—but chemotherapy is involved in the first 4 cycles. Were you able to get approval without the chemotherapy?
MADADI: We tried doing the chemotherapy; he had adverse events [AEs] from the first cycle and said no [to continuing chemotherapy].
MOHAMED: So you started with chemotherapy plus durvalumab plus tremelimumab, and then the intolerance to the chemotherapy pushed you to just continue the [immunotherapy] combination.
MADADI: Yes.
MOHAMED: What’s your experience with the tolerability?
MADADI: I was worried about the immune-mediated AEs, but he had more chemotherapy-related AEs with the first cycle [so] we had to hold it, and when I gave the immunotherapy it didn’t have many AEs.
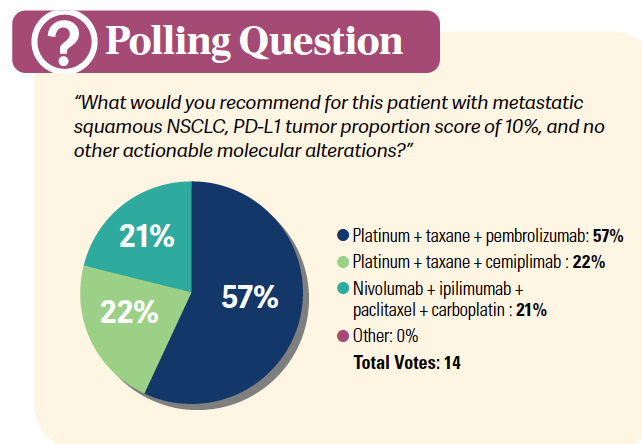
DISCUSSION QUESTION
- Which factors most influence your preferred regimen for a patient such as this?
MOHAMED: What factors most influence your preferred regimen for a patient such as this one? All of them are important, [including] efficacy, safety, tolerability, comorbidity, performance status, site of metastasis, burden of disease, PD-L1 expression, squamous vs nonsquamous, dosing and scheduling, or presence of non-EGFR actionable mutations like…KRAS G12C, [EGFR] exon 20 insertion, and STK11.
HOFFMAN: We’re missing the biggest factor [of] all: We didn’t ask the patient what he wants. We’re not treating ourselves. It’s what the patient wants. If this is a patient who says, “…I just want to be out of pain,” that’s a lot different than a younger patient who says, “In 18 months my son is getting married,” or “In 6 months, I’m having my first granddaughter….”
Comorbidities and performance status [are] important. In community medicine, we all know about the financial issues that are involved…[such as how] cisplatin used to be [affordable] and now it’s [more expensive]. What does the patient want and what toxicities is he willing to accept? In my practice…the patient walks in with a disease and their family, and that becomes the big difference.
MOHAMED: Great point, Dr Hoffman. Most of the time when we meet with the patient we would present all these factors and let the patient choose. When I said which factor influences the decision, it’s probably not how we’re going to decide. It’s [for] putting these factors in front of the patient and saying, “This is the efficacy, these are the toxicities, these are the logistics. Which way would you like to go?” and they make the decision at the end.
VARADI: I agree with Dr Hoffman, but it’s a very difficult question when you see the NCCN guidelines and you see 5, 6, or 7 variations. You always [must] know the patient’s circumstances and what the patient wants, what is their aim, and what is their social situation. Ultimately, we [must] give some kind of suggestion because relatively few patients understand all these treatments. We need to guide them, and…first of all, we look at the efficacy, the survival [outcomes], then what the patient can tolerate, because it’s very true that the patient suffers with the treatment, and then we also have to listen [to] their problems. It’s [also] important to see whether there are brain metastases and all those things.
HOFFMAN: My analogy when I talk to my fellows is that the NCCN guidelines are like GPS, but…you don’t follow GPS when you’re driving around your own neighborhood. It is the best we can do, but it’s certainly not written in solid concrete.
It comes out particularly with this patient who may do very well, because he doesn’t have any pulmonary symptomatology.… He’s not dealing with the [issues] that we normally deal with in patients with squamous cell carcinoma. He’s almost acting like [a patient with adenosquamous carcinoma] where adenocarcinoma plays an important role. The important thing for me here is what’s best for this patient, NCCN notwithstanding, because this is an [unusual] presentation of a squamous cell carcinoma and therefore we may have to think outside the box.
MOHAMED: Would anyone decide on the regimen based on the subset analysis, like KRAS mutation, STK11, or [EGFR] exon 20 insertion? Would that influence your decision when you make a choice? [Would you inform the] patient that there are these subset analyses and there may be different outcomes if they have this mutation on treatment with chemoimmunotherapy?
SHARMA: In nonsquamous patients with KRAS G12C and STK11 mutations, the subset analysis is very favorable to durvalumab plus tremelimumab plus 4 cycles of chemotherapy, so that should be [an option].
MOHAMED: I agree with you because most of [the data] are in adenocarcinoma, but again, we’re talking about squamous cell carcinoma.
SHARMA: But in squamous cell carcinoma, you don’t see KRAS G12C mutations.
MOHAMED: No, it’s a small percentage if you see it.
DISCUSSION QUESTIONS
- Considering the EMPOWER-Lung 3 (NCT03409614) data and the approval of cemiplimab plus chemotherapy as first-line therapy for advanced or metastatic NSCLC:
- Which data points are most impactful?
- Does this change your perception/opinion for using cemiplimab?
- When choosing systemic therapy for your patients, are you obligated to adhere to institutional guidelines?
MOHAMED: Which data points are most impactful for you when you look at the data from the EMPOWER-Lung 3 trial? What do you think about the data and how applicable are they to your patients?
HOFFMAN: My problems are hospitals. We’re never going to have head-to-head trials with the immuno-oncology agents. Every [regimen] that doesn’t come in first, loses. For the most part, when we’re dealing with NSCLC in our hospital system we’re using pembrolizumab, [because it was] the first out, [with the] best data initially. Even the…combination of nivolumab [Opdivo] and ipilimumab [Yervoy] takes second place, and it’s a distant second.
Right now, the way we can get pembrolizumab approved is very easy. The hospital carries it on shelf and the director of pharmacy…doesn’t want to bring something else in that’s going to cost the hospital money and not be used.
MOHAMED: Good point…. Back to the data, are these data different from what you saw with the other immunotherapy that has been on the market? What’s your perception of using it? Would that change your perception of using cemiplimab? The subset analysis in cemiplimab also showed that there are some similar results between squamous and nonsquamous,5 and we’ll always know patients with squamous histology don’t do as well as those with nonsquamous histology. Would that be a factor, if we are getting the same benefit in squamous vs nonsquamous?
OSEI-BOATENG: Comparing it with KEYNOTE-407 [NCT02775435], it looks a little better.5,6 The caveat is it might not be the same exact patient population. We’re looking at different trials, but it looks like the median progression-free survival and overall survival [are] a little bit better. The HRs are very impressive. They are [in the range of] 0.5 [From the Data5]. I’ve used cemiplimab in skin cancers and it’s very well tolerated with a good AE profile, so I would be comfortable using it. The data certainly look good. With the caveat of cost and some of the other things, if all things being equal, especially for squamous cell carcinoma, I haven’t used it, but I would. It’s not cumbersome. It’s the same every-3-week regimen. The [ipilimumab/nivolumab plus chemotherapy regimen from] CheckMate 9LA [NCT03215706] can be a bit cumbersome with the 2 immunotherapies and sequencing it, whereas most of us are comfortable doing it every 3 weeks with pembrolizumab and then going to monotherapy, and this is similar to that.

MOHAMED: For physicians who have used [cemiplimab] in squamous cell carcinoma of the skin, it’s a great drug that has been working good for us, [so] would that translate in any way to squamous cell carcinoma of the lung? We’re talking about a different disease here, but is that an additional comfort level? What do you think of the data in squamous cell carcinoma of the lungs that also looks good compared with the nonsquamous cell carcinoma, or at least similar, not inferior to the nonsquamous data?
MADALA: I’ve used single-agent cemiplimab in skin cancer and had great experience with 2 patients who were near hospice and completely turned around with complete response, and it was very well tolerated too. So I have great respect for cemiplimab. The data seem pretty good on this trial, especially with squamous cell carcinoma, but I take the subsets with a grain of salt, and we are creatures of habit. We’ve used pembrolizumab for [a long time] in the NSCLC setting, and we have protocols set in place in our practice [with] the formulary. We still use pembrolizumab, but I’d be comfortable using cemiplimab. [If it has a] better cost if it is available, cemiplimab is something I would be happy to use.
MOHAMED: Since you have it in the clinic for skin cancer…would you use it also in lung cancer?
MADALA: Right, absolutely.
MOHAMED: We have been using another drug for a long time, so changing the habit sometimes takes time for us.
REFERENCES
1. FDA approves cemiplimab-rwlc in combination with platinum-based chemotherapy for non–small cell lung cancer. FDA. November 8, 2022. Accessed September 22, 2023. https://tinyurl.com/yf3hyj4r
2. FDA approves tremelimumab in combination with durvalumab and platinum-based chemotherapy for metastatic non–small cell lung cancer. FDA. Updated November 18, 2022. Accessed September 22, 2023. https://tinyurl.com/5295aupx
3. Peters S, Cho BC, Luft A, et al. Association between KRAS/STK11/KEAP1 mutations and outcomes in POSEIDON: durvalumab±tremelimumab+chemotherapy in mNSCLC. J Thorac Oncol. 2022;17(suppl 9):S39-S41. doi:10.1016/j.jtho.2022.07.073
4. NCCN. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer, version 3.2023. Accessed September 22, 2023. https://tinyurl.com/ydsfxfaz
5. Makharadze T, Gogishvili M, Melkadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in advanced NSCLC: 2-year follow-up from the phase 3 EMPOWER-Lung 3 Part 2 trial. J Thorac Oncol. 2023;18(6):755-768. doi:10.1016/j.jtho.2023.03.008
6. Novello S, Kowalski DM, Luft A, et al. Pembrolizumab plus chemotherapy in squamous non–small cell lung cancer: 5-year update of the phase 3 KEYNOTE-407 study. J Clin Oncol. 2023;41(11):1999-2006. doi:10.1200/JCO.22.01990

Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More
Gholam Analyzes Treatment Outcomes for Advanced HCC in Child-Pugh B Population
April 28th 2024During a live Community Case Forum event in partnership with the Tennessee Oncology Practice Society, Pierre Gholam, MD, examined the current state of treatment for patients with hepatocellular carcinoma, looking in particular at what data is available for those with Child-Pugh B and C status who have poorer outcomes and have limited data from prospective clinical trials.
Read More