Lewis Breaks Down the Evolving Treatment Paradigm in HCC
During a Targeted Oncology™ Case-Based Roundtable™ event, Mark A. Lewis, MD, discussed the results of the SHARP, REFLECT, IMbrave150, and HIMALAYA trials of frontline regimens for patients with hepatocellular carcinoma.
Mark A. Lewis, MD
Director of Gastrointestinal Oncology
Intermountain Healthcare
Murray, UT

CASE SUMMARY
- A 64-year-old woman presented to her primary care physician with abdominal pain and fatigue.
- Medical history: body mass index, 31.0; type 2 diabetes
- α-Fetoprotein (AFP): 300 IU/mL
- Platelets: 230,000 cells/mcL
- Child-Pugh Class A
- Bilirubin: 1.4 mg/dL
- Albumin: 3.2 g/dL
- International normalized ratio: 2.1
- No hepatic encephalopathy
- Ascites not present
- Imaging: CT scan revealed 2 lesions in right hepatic lobe
(3 cm, 6 cm) with clear vascular involvement; no extrahepatic disease and no portal hypertension - Hepatitis B and C screening was negative
- Barcelona Clinic Liver Cancer stage: B
- ECOG performance status: 0
- The patient started on systemic therapy.
Targeted Oncology: What did the SHARP study (NCT00105443) add to the landscape of hepatocellular carcinoma (HCC) treatment?
LEWIS: This study got drilled into me during my fellowship. The SHARP study put sorafenib [Nexavar] on the map.1 This study had [602] patients with advanced HCC who had no prior treatment and were randomly assigned to sorafenib 400 mg twice daily or placebo. The primary outcomes were overall survival [OS] and time to symptomatic progression, and secondary outcomes were time to radiologic progression and safety.
What I find interesting is that the time to symptomatic progression…was shorter in sorafenib than it was in placebo, at 4.1 months vs 4.9 months, respectively [P = .77]. However, it was…OS at 10.7 months in the sorafenib arm vs 7.9 months in the placebo arm [HR, 0.69; 95% CI, 0.55-0.87; P < .001] that put sorafenib on the map, which is where it stayed for many years.
What was the purpose of the REFLECT trial (NCT01761266) that compared sorafenib with lenvatinib (Lenvima)?
The REFLECT trial was the attempt for lenvatinib to supplant sorafenib. It may be up to us to determine [whether] this was a true successor. The median progression-free survival [PFS] was essentially double in the lenvatinib arm vs the sorafenib arm, at 7.4 months vs 3.7 months, respectively [HR, 0.66;
95% CI, 0.57-0.77]. But for OS, there were very similar values between the 2 arms, at 13.6 months vs 12.3 months, respectively.2 The time to progression, which is related to PFS, was more than twice as long with lenvatinib than with sorafenib, at 8.9 months vs 3.7 months, respectively. The objective response rate [ORR] was almost 3-fold higher, with lenvatinib at 24% compared with sorafenib at 9%.
Why was the IMbrave150 trial (NCT03434379) of sorafenib vs atezolizumab (Tecentriq) plus bevacizumab (Avastin) such an important study?
The IMbrave150 study brought about a real paradigm shift after the years in the desert with sorafenib. This trial involved patients with HCC that was locally advanced or metastatic, unresectable, or both and who had received no prior treatment.3
They did stratify the patients by region—they thought Asia was an important demography and geography—then by ECOG performance status [0 vs 1], by AFP levels [for those with] less than 400 ng/mL vs [those with] 400 ng/mL or greater, and by the presence or absence of macrovascular invasion, extrahepatic spread, or both.3 The AFP stratification was bifurcated right at 400 ng/mL purposely. Patients were required to have a [esophagogastroduodenoscopy (EGD)] screening. Patients with untreated or incompletely treated varices with bleeding or with a high risk for bleeding were excluded, which may not reflect our patients in the real world.3
This study did lead to the FDA approval of this regimen as frontline therapy for unresectable HCC.4 There was a 2:1 randomization to atezolizumab and bevacizumab vs sorafenib, but it is important to note that the atezolizumab was given every 3 weeks at a dose of 1200 mg, and the bevacizumab was also given every 3 weeks at a dose of 15 mg/kg.3 The sorafenib dose was the same as in the SHARP trial, [at 400 mg twice daily].1
[These regimens were] given until the loss of clinical benefit or unacceptable toxicity, with coprimary end points of OS and investigator-assessed PFS per RECIST criteria.3 There was a lot of viral oncogenesis going on among the patients in this study. In fact, most patients [receiving the combination therapy] and [those receiving] sorafenib were dealing with one of the hepatitides.
The nonviral tier was [approximately] 30% of each arm.3 The efficacy and safety data were updated in the Journal of Hepatology last year, showing maybe not as long of a tail [on the survival curve] as we would have liked, but still showing a durable separation of the curves.5
The updated secondary efficacy outcomes were also reported. To the extent that things [such as] response matter to our patients or to us, there was almost a 3-fold greater ORR with atezolizumab and bevacizumab vs sorafenib [30% vs 11%, respectively], including some—although not enough—complete responses, [at 8% vs < 1%, respectively]….[Most] of these patients were still [experiencing] a response not [only] at the 1-year mark but also at the 18-month mark, which is remarkable and is a new milestone for the treatment of patients with HCC [Table3,5].
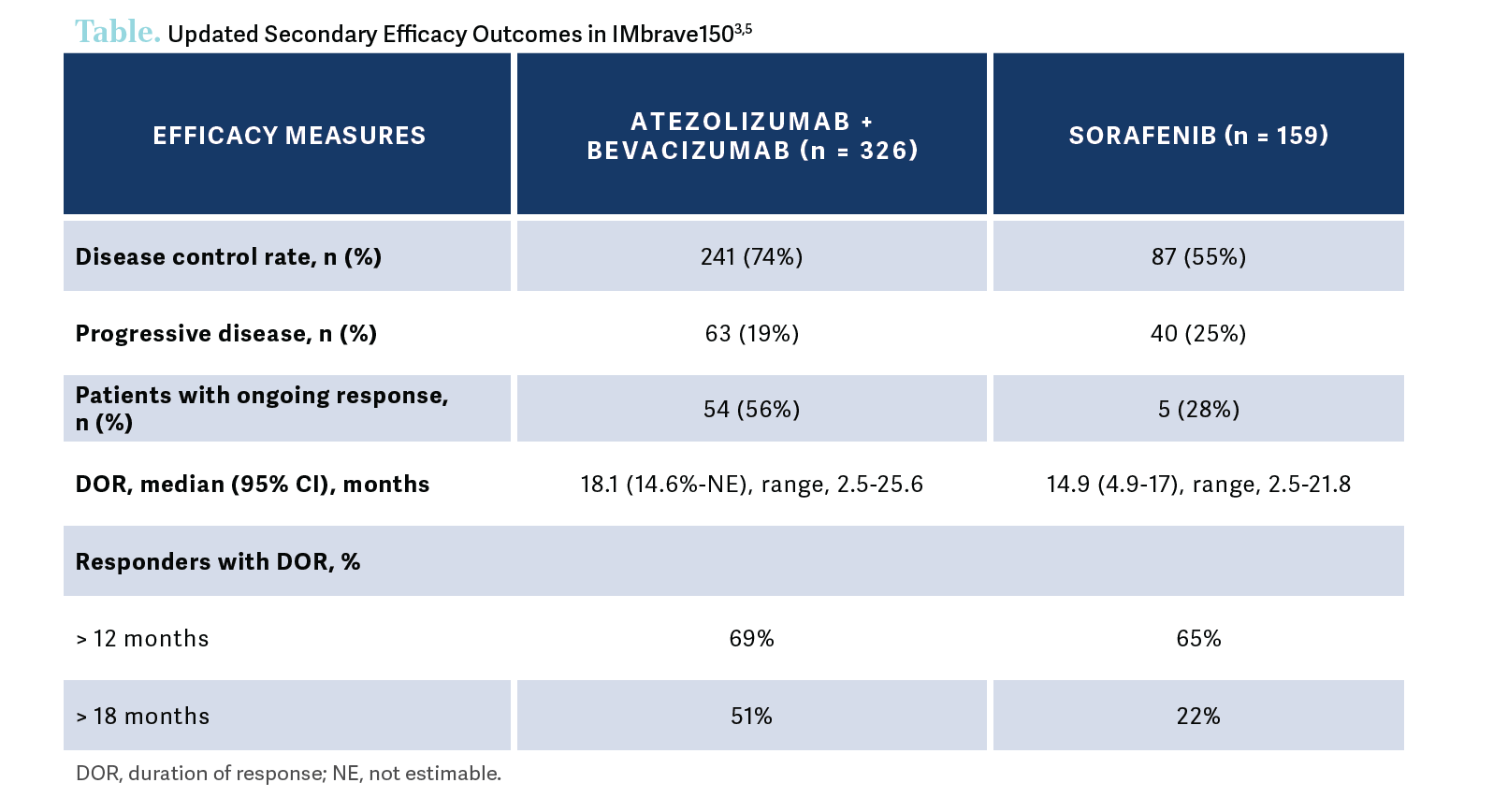
If a physician does not feel comfortable using bevacizumab, what alternatives exist that are backed by data?
If you don’t want to use bevacizumab, you do have the option of a durvalumab [Imfinzi]-based approach. The HIMALAYA study [NCT03298451] was a study of the combination of durvalumab plus tremelimumab-actl [Imjudo] vs single-agent sorafenib [or single-agent durvalumab] as first-line therapy. This study involved patients with untreated HCC who had a Child-Pugh score of A, a [Barcelona Clinic Liver Cancer] stage of B—so they weren’t eligible for locoregional therapy—or stage C, and no main portal vein thrombosis. Crucially, this study did not require an EGD. It started as a 4-arm study, but one of the arms closed, leaving us with the arm you might see referred to by the acronym STRIDE [Single Tremelimumab Regular Interval Durvalumab regimen], which is supposed to call out the fact that the tremelimumab was only given once in that arm.
Tremelimumab was given at a dose of 300 mg just once, so the anti–CTLA-4 exposure was minimal, and durvalumab was given monthly. In the arm that closed, patients were given tremelimumab 4 times at a lower dose. The primary objective was OS for durvalumab plus tremelimumab vs sorafenib, and the secondary objective was OS for durvalumab alone vs sorafenib.6 Clearly, the investigators were hoping they would find some synergy by combining the anti–CTLA-4 therapy and the anti–PD-1 therapy.
Again, it’s important to call out the HCC etiologies in the various arms. In this study, there was a balanced mix of viral and nonviral etiologies. Approximately two-thirds of the patients in each arm were dealing with an underlying viral hepatitis.6
What stood out to you about the STRIDE regimen?
The median OS for the STRIDE regimen was 16.4 months [95% CI, 14.2-19.6] vs 13.8 months [95% CI, 12.3-16.1] in the sorafenib arm [HR, 0.78; P = .0035]. It’s useful to note that there was a [30.7% vs 20.0%] 36-month OS rate in the combination and sorafenib arms, respectively, with the results again favoring the combination.7 The PFS curves of the STRIDE arm, the sorafenib arm, and the durvalumab arm were essentially superimposable.6 [However], I think the survival benefit is going to supplant this [result].
What safety data related to this treatment should physicians know about?
In the safety results, I don’t see a huge difference in adverse events [AEs] among the arms. Maybe you can argue that the durvalumab monotherapy was the least toxic, [with treatment-emergent AEs of any grade affecting 88.9% of patients], but I don’t see anything particularly salient in these results other than to point out that, with respect to the grade 3 or 4 immune-mediated AEs, the rate was [12.6%] in the STRIDE arm vs 6.2% for durvalumab alone and…[2.4%] in the sorafenib arm; you sometimes see this in placebo arms, too.6 It does make you wonder how that happens, mechanistically.
In the subgroup analysis, most subgroups favored durvalumab and tremelimumab over sorafenib. On the forest plot, there were a few [subgroups] that hewed to 1 and a [few] that crossed 1, but the results favored the dual immunotherapy [IO] approach for the most part. I thought it was interesting that patients with hepatitis B etiology seemed to benefit much more from the combination, whereas [it could be argued that] patients with hepatitis C etiology did not.7
Is there a trend with respect to how etiology affects the response of HCC to IO?
With respect to patients with nonviral HCC, [acknowledging that] cross-study comparisons are dangerous, [I would note that in a comparison of] CheckMate 459 [NCT02576509], KEYNOTE-240 [NCT02702401], and IMbrave150, the big takeaway is that for nonviral etiologies, we see a less impressive performance of IO vs control. When you consider the viral etiologies, hepatitis B in particular, I think there is more of a benefit.8 There was a lot of concern about immune reactivation of these viruses if patients with a viral etiology were to be exposed to an IO, yet we see that those patients may have an enhanced benefit in terms of oncologic outcome.8
Have the data from any of these studies been compared with data derived from real-world conditions?
It is always important to do that phase 4 work and look at what happens when these things are transported into our practices, [and that is what we see in] the AB-Real study. This was an international look at atezolizumab and bevacizumab in the clinics, and there was no discernable, meaningful difference between the survival curves [comparing IMbrave150 data with real-world data]. I thought this was rather amusing.
There were a couple points along the survival curves that separated, but for the most part, these curves are very similar. The median OS and median PFS values were also quite comparable for the AB-Real and IMbrave150 studies [for OS, 15.74 months vs 19.20 months, respectively; for PFS, 6.91 months vs 6.91 months, respectively], especially when you consider the HRs [for OS at 0.87 and PFS at 0.90].9
References
1. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi:10.1056/NEJMoa0708857
2. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi:10.1016/S0140-6736(18)30207-1
3. Finn RS, Qin S, Ikeda M, et al; IMbrave Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
4. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. FDA. Updated June 1, 2020. Accessed April 21, 2023. https://bit.ly/3CbMtOc
5. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862-873. doi:10.1016/j.jhep.2021.11.030
6. Abou-Alfa GK, Lau G, Kudo M, et al; HIMALAYA Investigators. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8). doi:10.1056/EVIDoa2100070
7. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(suppl 4):379. doi:10.1200/JCO.2022.40.4_suppl.379
8. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450-456. doi:10.1038/s41586-021-03362-0
9. Fulgenzi CAM, Cheon J, D’Alessio A, et al. Observational registry of atezolizumab plus bevacizumab use in routine clinical practice: preliminary results of the AB-Real international study. Presented at: International Liver Cancer Association Annual Conference 2022; September 1-4, 2022; Madrid, Spain. Accessed April 21, 2023. https://bit.ly/3WK6PaR

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More