Adagrasib Reveals Efficacy in KRAS G12CMutated NSCLC
Updated findings from the early-stage KRYSTAL-1 study showed that treatment with adagrasib resulted in durable responses, broad disease control, and provided extensive predicted coverage throughout the dosing interval in patients with KRAS G12Cmutant advanced non–small cell lung cancer.
Gregory J. Riely, MD, PhD
Updated findings from the early-stage KRYSTAL-1 study (NCT03785249) showed that treatment with adagrasib (MRTX849) resulted in durable responses, broad disease control, and provided extensive predicted coverage throughout the dosing interval in patients with KRAS G12Cmutant advanced non–small cell lung cancer (NSCLC). Results were presented during the European Lung Cancer Virtual Conference 2021.1
Data showed that the 600-mg, twice-daily dose of adagrasib, a selective inhibitor of KRAS G12C, yielded an overall response rate (ORR) of 43% and 45% in the trial’s phase 1/1b (n = 14) and phase 1/1b Data showed that the 600-mg, twice-daily dose of adagrasib, a selective inhibitor of KRAS G12C, yielded an overall response rate (ORR) of 43% and 45% in the trial’s phase 1/1b (n = 14) and phase 1/1b.
The disease control rate was 100% in the phase 1/1b population and 96% in the phase 1/1b and 2 cohort. In total, 57% and 51% of patients, respectively, had stable disease. Disease progression did not occur in the phase 1/1b cohort but did occur in 2% of those in the phase 2 pooled cohort.
Pharmacokinetic (PK) data indicated that adagrasib yielded a Cave of 2.63 µg/mL, which is 2- to 5-fold above the target threshold for the full dosing interval. More-over, investigators found that the Cave PK parameter was best matched to nonclinical antitumor activity. Additionally, the extensive volume of distribution was predicted based on nonclinical studies.
“The observed steady-state concentration was above the target threshold for the full dosing interval,” lead study author Gregory J. Riely, MD, PhD, vice chair of clinical research in the Department of Medicine at Memorial Sloan Kettering Cancer Center in New York, New York, said in a virtual presentation during the meeting. “We also observed low peak to trough ratio at 1.27 and a half-life of 24 hours.”
Adagrasib is a covalent inhibitor of KRAS G12C that irreversibly and selectively binds KRAS G12C in its inactive guanosine diphosphate (GDP)–bound state, according to Riely. The agent has been optimized for desired properties, demonstrating high selectivity for KRAS G12C mutations over wild-type and favorable PK properties, including oral bioavailability, a half-life of approximately 24 hours, and extensive tissue distribution.
By maintaining a continuous exposure to adagrasib above the target threshold, investigators theorized, inhibition of KRAS-dependent signaling will be enabled for the complete dosing interval, which could maximize depth and duration of the agent’s antitumor activity.
"KRAS G12C mutations act as an oncogenic driver and occur in approximately 14% of patients with NSCLC,” Riely said. “The KRAS protein cycles between GTP-on and GDP-off states and has a protein resynthesis of approximately 24 hours.”
To be eligible for enrollment, patients had to have KRAS G12C–mutant unresectable or metastatic NSCLC following progression on or after treatment with a PD-1/PD-L1 inhibitor in combination with or following chemotherapy.
In the phase 1, dose-escalation portion of the trial, investigators examined various once-daily doses of adagrasib: 150, 300, 600, and 1200 mg. They also looked at 600 mg given twice daily, which moved to the expansion phase and became the recommended phase 2 dose. Clinical findings indicated that the 600-mg, twice-daily dose of adagrasib in the phase 1/1b portion had a clinical benefit rate of 96%.
The phase 1b section looked at adagrasib alone and combined with pembrolizumab (Keytruda), afatinib (Gilotrif), and cetuximab (Erbitux). The phase 2 portion looked at adagrasib alone in patients with NSCLC (n = 61), colorectal cancer, and other solid tumors.
The primary end points of the phase 1b study consisted of safety, maximum-tolerated dose, PK, and the recommended phase 2 dose, as well as ORR in the phase 2 portion.
Secondary end points in the phase 1b portion included ORR, duration of response, progression-free survival, and overall survival. Secondary outcome measures in the phase 2 portion included safety.
Most patients across all phases were current or former smokers (89% and 95%, respectively) and had nonsquamous histology (100% and 96%). Patients had an average of 3 (range, 1-9) and 2 (range, 1-9) prior lines of therapy, respectively, and the majority of patients had previously received a PD-1/PD-L1 inhibitor (89% and 92%).
After a median follow-up of 9.6 months, 5 of 6 responders remain on treatment, which has been ongoing for more than 11 months. The median time to response was 1.5 months, and the median duration of treatment was 8.2 months (range, 1.4 to 13.1+).
“We see some clear evidence that we’re inhibiting KRAS signaling. The gene set enrichment analysis [identified] downregulation of KRAS signaling and downregulation of MYC, E2F, G2M, and MTORC1,” Riely explained. “We’re seeing a trend, but it’s hard to determine significance based on this small number of samples.”
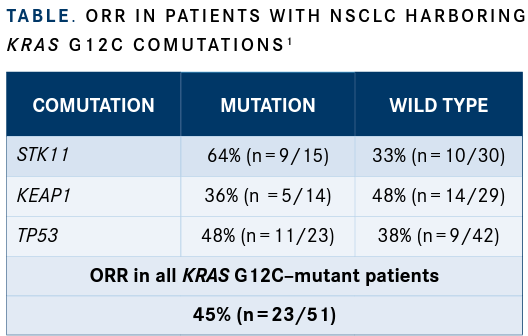
Investigators also conducted a preliminary analysis examining comutations with KRAS G12C. Baseline next-generation sequencing reports were reviewed in order to conduct the analysis for all patients with NSCLC who had available mutation data. Notably, patients who had KRAS G12C mutations and an STK11 comutation experienced an ORR of 64%, although there were no apparent trends with KEAP1, TP53, or other common mutations and response rate (TABLE1).
“As many know, comutations in STK11 and KEAP1 are thought to predict lack of benefit from immunotherapy,” Riely said. “We decided to look at response rate in this cohort of patients.”
Regarding safety for all patients treated at the 600-mg, twice-daily dose (n = 110), the most common all-grade, treatment-related adverse effects (TRAEs) included nausea (54%), diarrhea (51%), vomiting (35%), and fatigue (32%). Grade 3/4 TRAEs included fatigue (6%), increased alanine aminotransferase (5%), aspartate aminotransferase (5%), and QT prolongation (3%). There were 2 grade 5 TRAEs: pneumonitis, which developed in a patient with recurrent pneumonitis, and cardiac failure. In total, TRAEs led to discontinuation in 4.5% of patients.
“Adagrasib is a KRAS G12C–selective covalent inhibitor with a long half-life and extensive predictive target coverage throughout the dosing interval,” Riely concluded. “[It] is well tolerated and provides durable response and broad disease control to [this patient population].”
REFERENCE
1. Riely GJ, Ignatius Ou SH, Rybkin II, et al. KRYSTAL-1: Activity and pre-liminary pharmacodynamic (PD) analysis of adagrasib (MRTX849) in pa-tients (pts) with advanced non-small-cell lung cancer (NSCLC) harboring KRASG12C mutation. Presented at: European Lung Cancer Virtual Confer-ence 2021; March 25-27, 2021. Abstract 99O_PR
Brown Examines Lenvatinib/Pembrolizumab Combination in pMMR Endometrial Cancer
May 17th 2024During a Case-Based Roundtable® event, Jubilee Brown, MD, discussed the efficacy and safety data from the KEYNOTE-775/Study 309 trial of lenvatinib plus pembrolizumab in patients with mismatch repair proficient advanced endometrial cancer.
Read More