Novel and Emerging Therapies for Melanoma
Until recently, the cornerstone of therapy for metastatic melanoma had been chemotherapy with dacarbazine (DTIC) and immunotherapy with high-dose interleukin-2 (HD IL-2) or interferon- (IFN- ).
FIGURE 1. Selected Pathways in Melanoma
Selected Pathways in Melanoma
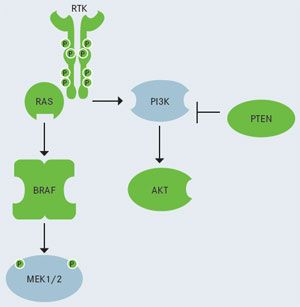
RTK, receptor tyrosine kinase signaling pathway, which includesBRAFandNRASoncogenes.
PI3K, phosphatidylinositol 3-kinase (pathway activation also occurs in melanoma.)
Until recently, the cornerstone of therapy for metastatic melanoma had been chemotherapy with dacarbazine (DTIC) and immunotherapy with high-dose interleukin-2 (HD IL-2) or interferon- (IFN- ). Although considered the standard of care, DTIC has shown objective response rates as low as 15.3% for a total of 3356 patients enrolled in more than 20 studies. Additionally, most of these responses were partial. Despite significant toxicity, immunotherapy with HD IL-2 resulted in a substantial benefit for some patients, leading to a 27% survival rate at 2 years and 16% at 4 years, in a trial involving 684 patients. Additionally, the efficacy of IFN- remains controversial. Its significant toxicity, especially at high doses, has restricted its use. Due to its easier use and reduced side effects compared with unmodified IFN- , pegylated IFN- was approved by the FDA in the adjuvant setting in 2011 for patients with lymph node-positive resected melanoma. Trials remain ongoing to evaluate its optimal dosage, as well as the subset of patients that might benefit from this option.1
Frontline treatment options for patients with advanced melanoma have recently improved with the 2011 FDA approval of vemurafenib and ipilimumab, both with indications for metastatic disease, and other therapies are in development (Figure 1).
Vemurafenib is an orally available, targeted agent that selectively inhibits the BRAF kinase. It has specificity for theV600E-mutated form of the protein, the oncogenically active variant in 90% of patients withBRAF-mutated melanoma.2In phase II clinical trials in individuals withBRAF-mutant metastatic melanoma, vemurafenib induced high antitumor activity and improvement in survival. Subsequently, in the randomized phase III BRIM-3 study, vemurafenib was compared with DTIC in 675 patients with previously treated,BRAF V600E-positive metastatic melanoma. The response rate was substantially improved in patients treated with vemurafenib versus DTIC (48% vs 5%). Patients in the vemurafenib arm also experienced a markedly improved 6-month overall survival (OS) compared with those in the DTIC arm (84% vs 64%). Vemurafenib was also associated with a relative reduction in risk of death (63%), and a 74% reduction in the risk of either death or disease progression compared with DTIC (P< .001).3,4
By the median follow-up of 3.8 months (2.3 months in the DTIC-treated group), all OS and progression-free survival (PFS) endpoints had been met, and patients in the DTIC arm were crossed over to vemurafenib. The most frequent grade 2 adverse events with vemurafenib treatment included arthralgia, fatigue, and rash. The most common grade 3 adverse event was cutaneous squamous cell carcinoma (SCC), occurring in 24% of patients, with a median time to first appearance of between 7 and 8 weeks. Risk factors for the development of cutaneous SCC while receiving vemurafenib include older age (≥ 65 years), prior skin cancer, and chronic sun exposure. Patients therefore require screening prior to vemurafenib treatment, followed by screening every 2 months while on therapy.3,4
Ipilimumab is a fully human, monoclonal antibody directed against the T-cell antigen cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) (Figure 2). In a multicenter, single-arm, phase II clinical trial, ipilimumab was shown to be active in patients with relapsed metastatic melanoma, with 27% of patients achieving disease control, despite a low overall response rate (5.8%). The 1-year and 2-year survival rates were 47.2% and 32.8%, respectively.5
FIGURE 2. Ipilimumab Blocks CTLA-4: T-Cell Activation
Ipilimumab Blocks CTLA-4: T-Cell Activation
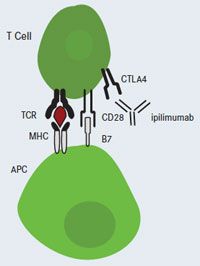
APC, antigen-presenting cell; CTLA-4, cytotoxic T-lymphocyte antigen-4; MHC, major histocompatibility complex; TCR, T-cell receptor.
Subsequently, the randomized, prospective phase III MDX010-20 trial evaluated ipilimumab in 676 patients with previously treated metastatic melanoma. Ipilimumab significantly prolonged median OS in patients when administered either as a single agent (10.1 months) or combined with the gp100 vaccine (10.0 months), versus gp100 alone (6.4 months;P= .003 andP< .001 for each comparison, respectively). Two-year OS rates were 23.5%, 21.6%, and 13.7% for single-agent ipilimumab, ipilimumab combined with gp100, and gp100 alone, respectively. Additionally, some patients with disease progression following treatment benefited from re-induction with ipilimumab.6
Another phase III trial, Study 024, evaluated a higher dose of ipilimumab (10 mg/kg) in 502 patients with previously untreated metastatic melanoma. Patients were randomized to receive either DTIC plus placebo, or DTIC combined with ipilimumab. OS was significantly improved in patients in the DTIC plus ipilimumab combination group (11.2 vs 9.1 months;P< .001). Survival rates were higher in the ipilimumab DTIC group at 1 year (47.3% vs 36.3%), 2 years (28.5% vs 17.9%), and 3 years (20.8% vs 12.2%). Treatment with ipilimumab plus DTIC produced a two-fold improvement in the median duration of response compared with the DTIC-plus-placebo arm (19.3 vs 8.1 months,P= .03). Grade 3 or 4 adverse events occurred in 56.3% of patients treated with DTIC plus ipilimumab, compared with 27.5% in those treated with DTIC and placebo (P< .001).7
Recent Approvals
Both vemurafenib and ipilimumab are recommended by the National Comprehensive Cancer Network (NCCN) as category 1 options for treatment of advanced melanoma. The choice of treatment is individualized: Since patients with less aggressive, low volume, or asymptomatic metastatic melanoma may have more time to allow an antitumor immune response to mount, they may be good candidates for ipilimumab therapy; patients with more aggressive,BRAFmutation-positive advanced melanoma, however, may benefit more from first-line treatment with vemurafenib.8Dabrafenib, a BRAF inhibitor, received approval for the treatment of patients with unresectable or metastatic melanoma withBRAF V600Emutation in late May 2013. A multicenter, international, open-label, randomized, active-controlled phase III trial evaluated the efficacy and safety of dabrafenib compared with DTIC in 250 patients with previously untreated, histologically confirmed, unresectable stage III or IV melanoma that was determined to beBRAF V600Emutation-positive by centralized testing. Median PFS was significantly improved in patients in the dabrafenib arm compared with the DTIC arm (5.1 vs 2.7 months). The most common drug-related AEs were hyperkeratosis, headache, pyrexia, arthralgia, papilloma, alopecia, and palmar-plantar erythrodysesthesia syndrome. Serious AEs involved development of new primary skin cancers (cutaneous SCC, new primary melanomas, and keratoacanthomas), febrile drug reactions requiring hospitalization, hyperglycemia, and uveitis/iritis.9
Trametinib is a MEK inhibitor and first in its class to receive approval, also in late May 2013, and is indicated for use in patients with unresectable or metastatic melanoma withBRAF V600EorV600Kmutation. A multicenter, international, open-label, randomized active-controlled phase III trial evaluated the efficacy and safety of trametinib compared in 322 patients with histologically confirmed, stage IIIc or IV melanoma determined to beBRAF V600EorV600Kmutation-positive by centralized testing. Patients were not permitted to have received more than one prior chemotherapy regimen, and those with previous exposure to BRAF inhibitors or MEK inhibitors were ineligible. Median PFS was significantly improved in patients in the trametinib arm, compared with those receiving chemotherapy (4.8 vs 1.5 months). Trametinib did not demonstrate antitumor activity in patients who had received prior BRAF inhibitor therapy. The most common drugrelated AEs were rash, diarrhea, and lymphedema. Serious AEs included cardiomyopathy, retinal pigment epithelial detachment, retinal vein occlusion, interstitial lung disease, and serious skin toxicity.10
References
- Pretto F, Neri D. Pharmacotherapy of metatstatic melanoma: emerging trends and opportunities for a cure. Pharmacol Ther. May 2, 2013 [Epub ahead of print].
- Nikolaou VA, Stratigos AJ, Flaherty KT, et al. Melanoma: new insights and new therapies. J Invest Derm. 2012;132:854-863.
- Evans MS, Madhunapantula SV, Robertson GP, et al. Current and future trials of targeted therapies in cutaneous melanoma. WS El-Deiry (ed), Impact of genetic targets on cancer therapy. Adv Exp Med Bio. 2013;223-255.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21(8):1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517-2526.
- National Comprehensive Cancer Network. Melanoma. Clinical Practice Guidelines in Oncology. 2012. Available at: http:// www.nccn.org/professionals/physician_gls/f_guidelines. asp#melanoma. Accessed June 16, 2013.
- FDA. Darafenib. 2013. Available at: http://www.fda.gov/Drugs/ InformationOnDrugs/ApprovedDrugs/ucm354477.htm. Accessed June 16, 2013.
- FDA. Trametinib. 2013. Available at: http://www.fda.gov/ Drugs/InformationOnDrugs/ApprovedDrugs/ucm354478.htm. Accessed June 16, 2013.