Novel Agents May Challenge Standard Therapy in Advanced HER2+ Breast Cancer
The treatment paradigm for HER2-positive breast cancer may be reshaped over the next several years, as head-to-head studies comparing new agents to the current standard of care begin to yield results, said Sara A. Hurvitz, MD.
Sara A. Hurvitz, MD

Sara A. Hurvitz, MD
The treatment paradigm for HER2-positive breast cancer may be reshaped over the next several years, as head-to-head studies comparing new agents to the current standard of care begin to yield results, said Sara A. Hurvitz, MD.
Newer agents currently being evaluated in clinical trials include the oral tyrosine kinase inhibitors (TKIs) neratinib (Nerlynx) and tucatinib (ONT-380), the antibodydrug conjugate trastuzumab deruxtecan (DS-8201), and the monoclonal antibody margetuximab (MGAH22), said Hurvitz, director of Breast Cancer Clinical Research and an assistant professor of medicine at David Geffen School of Medicine at the University of California, Los Angeles.
During a presentation on current and new strategies in the treatment of advanced HER2-positive disease at the36thAnnualMiami Breast Conference®(MBCC), Hurvitz reviewed key data involving these drugs and other emerging agents.
“Head-to-head studies comparing some of these newer agents to standard of care are just beginning,” Hurvitz said in an interview in advance of her presentation. “So in anywhere between 5 and 10 years, 1 or 2 of these molecules may challenge the frontline or second-line standard that we have today, if their benefits and toxicity profile look better than our standard therapy.”
The impetus for developing new HER2-targeting strategies is driven by the need to overcome treatment resistance, which arises in most patients who take trastuzumab (Herceptin), Hurvitz said in her conference abstract. Although multiple HER2-directed therapies are available, “the pace of discovery has sped up in the last several years, resulting in the appearance of multiple novel agents entering clinical trials, many with promising results in early-phase testing,” Hurvitz said.
Hurvitz noted in the interview that the current firstline standard of care for HER2-positive metastatic breast cancer remains dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab plus chemotherapy. The CLEOPATRA study showed that adding pertuzumab to trastuzumab and docetaxel improved median overall survival (OS) by almost 16 months compared with placebo plus trastuzumab and docetaxel. The triplet extended median OS to 56.5 months versus 40.8 months with the standard therapy (HR, 0.68; 95% CI, 0.56-0.84;P= .0002).1
“For the majority of patients, that should be offered frontline,” Hurvitz said. “There are a lot of controversies in the management of breast cancer itself, but this is one area that’s not controversial.”
Likewise, in the second-line setting after progressing on trastuzumab-based therapy, patients should receive ado-trastuzumab emtansine (T-DM1; Kadcyla), she said. In the phase III EMILIA trial comparing the antibodydrug conjugate or lapatinib (Tykerb) plus capecitabine, T-DM1 extended median OS by 5.8 months (30.9 months vs 25.1 months, respectively). The HR for death from any cause was 0.68 [95% CI, 0.55-0.85;P<.001], and was associated with fewer adverse effects.2Hurvitz noted that lapatinib-based therapies continue to be used after T-DM1.
Neratinib
In terms of development, the most advanced of the new HER2 options is neratinib, which was approved in July 2017 for extended adjuvant treatment of adults with early-stage HER2-positive breast cancer following postoperative trastuzumab. In the ExteNET trial, the rate of invasive disease-free survival (iDFS) at 24 months was 94.2% in patients treated with neratinib compared with 91.9% in those receiving placebo (HR, 0.66; 95% CI, 0.49-0.90;P= .008).3
The benefit with neratinib was more pronounced among patients with hormone receptorpositive disease, with an iDFS of 95.6% versus 91.5% with placebo. In terms of lymph nodes, patients with ≥4 positive nodes experienced a higher rate of iDFS benefit with neratinib versus placebo (91.4% vs 87.3%, respectively) than did those with negative nodes or 1 to 3 positive nodes.3
The phase III NALA trial is comparing neratinib plus capecitabine with lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer who failed 2 or more prior lines of HER2-directed therapy. Overall, 621 patients have been randomized 1:1 to receive oral neratinib at 240 mg once daily plus capecitabine at 1500 mg/m2 daily in 2 doses or oral lapatinib at 1250 mg once daily combined with capecitabine at 2000 mg/m2 daily in 2 doses.
The neratinib regimen led to a statistically significant improvement (P= .0059) in centrally confirmed progression-free survival (PFS) compared with lapatinib and capecitabine, according to Puma Technology, the manufacturer of neratinib.4The neratinib combination also improved overall survival, but the difference was not found to be statistically significant (P= .21), the company said.
Full findings of the study will be presented at an upcoming medical meeting, the company said. The results also will be submitted to the FDA and other regulatory agencies for approval in this patient population.
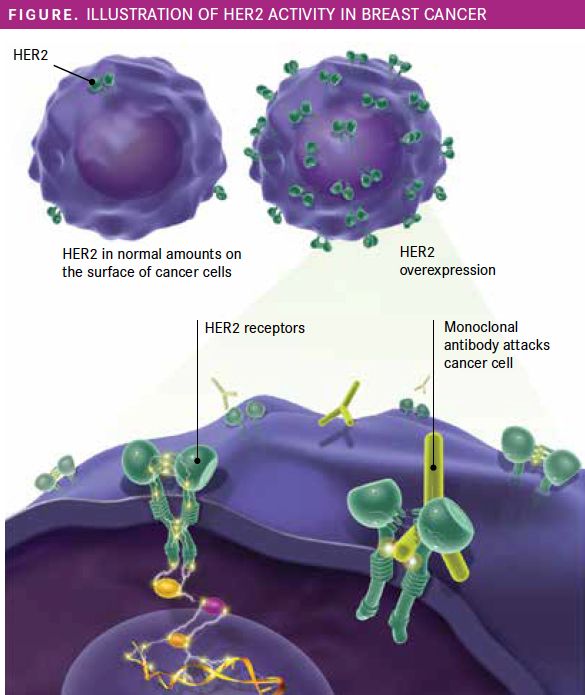
Figure. Illustration of HER2 Activity in Breast Cancer
Figure. Illustration of HER2 Activity in Breast Cancer
Drugs in the Pipeline
Trastuzumab deruxtecan
Other potentially transformative novel agents are at earlier stages of development but are already stoking excitement in the breast cancer research community.
Trastuzumab deruxtecan has an FDA breakthrough therapy designation for patients with HER2-positive, locally advanced, or metastatic breast cancer who have been treated with trastuzumab and pertuzumab and have disease progression after T-DM1.
In a phase I study in this patient population, trastuzumab deruxtecan demonstrated a confirmed overall response rate (ORR) by RECIST 1.1 criteria of 54.5% among 99 evaluable patients with HER2-positive breast cancer and 50.4% among 34 participants with HER2-low disease, according to findings presented at the 2018 San Antonio Breast Cancer Symposium. HER2 positivity was defined as immunohistochemistry (IHC) 3+ or in situ hybridization (ISH) positive. Breast cancer was considered HER2 low with expression of IHC 1+ or 2+ with negative ISH results.5
“We’re seeing objective response rates north of 50% in very heavily pretreated breast cancer. [Those are] extraordinary data,” Hurvitz said. A phase II study of trastuzumab deruxtecan in HER2-positive breast cancer has completed enrollment, 3 phase III randomized trials have been launched, and a number of phase I studies are under way, she said.
Tucatinib
Like lapatinib and neratinib, tucatinib is an oral TKI. However, tucatinib blocks just HER2, causing fewer off-target effects such as rash and diarrhea than the other drugs, which target multiple receptors.
In phase IB results from a nonrandomized, open-label study, 83% (5 of 6) of patients with measurable disease treated with tucatinib plus capecitabine had an objective response, as did 40% (6 of 15) receiving tucatinib plus trastuzumab. Among patients treated with the combination of all 3 drugs, 61% (14 of 23) had an objective response.6
The drug also showed activity in patients with brain metastases. In an exploratory analysis of all the combinations, a brain-specific response was observed in 5 of 12 patients (42%) with brain metastases who were treated with the recommended phase II dose of 300 mg twice a day of tucatinib.6
“That drug is not only exciting because its preclinical activity looks good as a single agent and in combination with trastuzumab and capecitabine and with T-DM1 but, importantly, [also because] it crosses the bloodbrain barrier, and we’re seeing objective responses in the brain in the phase I studies,” Hurvitz said. Up to 50% of patients with metastatic breast cancer will also have brain metastases.
The phase II HER2CLIMB trial is evaluating tucatinib or placebo in combination with capecitabine and trastuzumab in patients with progressive unresectable locally advanced or metastatic HER2-positive breast cancer who had prior treatment with trastuzumab, pertuzumab, and T-DM1 (NCT02614794).
Margetuximab
Another investigational agent with the potential to alter treatment of some advanced HER2-positive breast cancers is margetuximab, an Fc-optimized monoclonal antibody directed at HER2. It has been described as an optimized version of trastuzumab and it has an FDA fasttrack designation.
In the phase III SOPHIA trial (NCT02492711), the combination of margetuximab plus chemotherapy improved PFS compared with trastuzumab plus chemotherapy in heavily pretreated patients with metastatic HER2-positive disease, according to MacroGenics, the company developing the drug.7
Patients in the margetuximab arm experienced a 24% reduction in the risk of disease progression or death versus patients in the trastuzumab arm (HR, 0.76;P= .033). In a subpopulation of patients who are carriers of the CD16A (FcγRIIIa) 158F allele, which has been linked to a lower clinical response to HER2-targeting therapies, there was a 32% reduction in PFS win the margetuximab arm compared with the trastuzumab arm (HR, 0.68;P= .005).
“There are currently no approved agents for the treatment of patients with metastatic HER2-positive breast cancer who have previously received trastuzumab, pertuzumab, and adotrastuzumab emtansine,” Hope S. Rugo, MD, an MBCC cochair and 1 of the leading investigators on the SOPHIA trial, said in a statement.
“If margetuximab is approved, based on SOPHIA data, I believe that this agent could become a valuable treatment option for these patients,” added Rugo, who is a professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center.
The company plans to submit the SOPHIA findings to the FDA during the second half of 2019 in support of a biologics license application for margetuximab.
Pyrotinib
Another agent Hurvitz is watching is pyrotinib, an irreversible pan-HER receptor TKI that has shown antitumor activity in HER2-positive metastatic breast cancer. In a phase I study conducted in China, 18 (50%) of 36 patients had a partial response, 4 (11.1%) had stable disease ≥24 weeks, and 7 (19.4%) had progressive disease.8
The best ORR among patients previously treated with trastuzumab was 33.3%, compared with 83.3% in trastuzumab-naïve patients. The incidence of diarrhea of any grade was relatively high at 44%; grade 3 diarrhea was a dose-limiting toxicity for 2 patients who received a higher dose of the drug. Pyrotinib was conditionally approved in China last year for use in in combination with capecitabine for patients with HER2-positive advanced or metastatic disease. It is being studied in several trials, including a phase I trial in solid tumors that is recruiting in the United States (NCT02500199).
Palbociclib Combination
Among the other studies on Hurvitz’s radar is an ongoing phase II trial of the CDK4/6 inhibitor palbociclib (Ibrance) in combination with trastuzumab in patients with advanced estrogen receptor (ER)positive/HER2-positive breast cancer. In preliminary results from the SOLTI-1303 PATRICIA study, 19 of 45 patients in 3 cohorts remained progression free at 6 months with the combination, with a higher median PFS in those with luminal versus nonluminal breast cancer (12.4 months vs 4.1 months; adjusted HR, 0.30;P= .025).9
Atezolizumab Regimen
Hurvitz also noted the KATE2 trial, which is testing the combination of T-DM1 with the antiPD-L1 immunotherapy agent atezolizumab (Tecentriq) in patients with previously treated HER2-positive advanced disease.10
Early results show an ORR of 45% and a median PFS of 8.2 months for those who received the combination versus 43% and 6.7 months, respectively, for those treated with T-DM1 plus placebo. The study did not find clinically significant PFS benefit (HR, 0.82; 95% CI, 0.55-1.23;P= .3332); OS and duration of response data were not yet mature.
References:
- Perjeta [prescribing information]. South San Francisco, CA: Genentech, Inc; 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/125409s123lbl.pdf. Accessed February 27, 2019.
- Verma S, Miles D, Gianni L, et al; EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer.N Engl J Med. 2012;367(19):1783-1791. doi: 10.1056/NEJMoa1209124.
- Nerlynx [prescribing information]. Los Angeles, CA: Puma Biotechnology, Inc; 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208051s002lbl.pdf. Accessed February 25, 2019.
- Puma Biotechnology announces top line results of the phase III NALA trial of neratinib in patients with HER2-positive metastatic breast cancer [news release]. Los Angeles, CA: Puma BioTechnology, Inc; December 17, 2018. www.pumabiotechnology.com/pr20181217.html. Accessed February 19, 2019.
- Modi S, Tsurutani J, Tamura K, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: updated results of a large phase 1 study. Presented at: 2018 San Antonio Breast Cancer Symposium; December 4-8, 2018; San Antonio, TX. Abstract Abstract P6-17-02. abstracts2view.com/sabcs/view.php?nu=SABCS18L_486&terms=.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803-1813.
- Murthy R, Borges VF, Conlin A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study.Lancet Oncol. 2018;19(7):880-888. doi: 10.1016/S1470-2045(18)30256-0.
- MacroGenics announces positive results from pivotal phase 3 SOPHIA study of margetuximab [news release]. Rockville, MD: MacroGenics, Inc; February 6, 2019. ir.macrogenics.com/news-releases/news-release-details/macrogenics-announces-positive-results-pivotal-phase-3-sophia. Accessed February 21, 2019.
- Ma F, Li Q, Chen S, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer.J Clin Oncol. 2017;35(27):3105-3112. doi: 10.1200/JCO.2016.69.6179.
- Ciruelos E, Villagrasa P, Oliveira M, et al. SOLTI-1303 PATRICIA phase II trial (stage I)palbociclib and trastuzumab in postmenopausal patients with HER2-positive metastatic breast cancer. Presented at: 2018 San Antonio Breast Cancer Symposium; December 4-8, 2018; San Antonio, TX. Abstract PD3-03. abstracts2view.com/sabcs18/view.php?nu=SABCS18L_1427&terms=.
- Emens LA, Esteva F, Beresford M, et al. Results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC). Presented at: 2018 San Antonio Breast Cancer Symposium; December 4-8, 2018; San Antonio, TX. Abstract PD3-01. abstracts2view.com/sabcs/view.php?nu=SABCS18L_1253&terms=.