KEYNOTE-001 Analysis Supports Pembrolizumab Use in Advanced Melanoma
At the 2015 ASCO Annual Meeting, Adil I. Daud, MD, UCSF Hellen Diller Family Comprehensive Cancer Center, presented a pooled analysis of 655 patients with advanced melanoma enrolled in the KEYNOTE-001 trial.
Adil I. Daud, MD

Adil I. Daud, MD
More than 2000 patients have been treated with pembrolizumab (PEM) in KEYNOTE trials 001, 002, and 006. At the 2015 ASCO Annual Meeting, Adil I. Daud, MD, clinical professor in the Department of Medicine (Hematology/Oncology), University of California, San Francisco, and director, Melanoma Clinical Research, UCSF Hellen Diller Family Comprehensive Cancer Center, presented a pooled analysis of 655 patients with advanced melanoma enrolled in the KEYNOTE-001 trial.1
Daud described the four cohorts comprising the 001 trial, and hence the data available for the present analysis: (1) a nonrandomized cohort of ipilimumab-naïve (IPI-N) and ipilimumab-treated (IPI-T) patients testing doses of PEM, 2 mg/kg every 3 weeks, 10 mg/kg every 3 weeks, and 10 mg/kg every 2 weeks (n = 135); (2) a randomized trial of IPI-T patients testing PEM at 1 mg/kg every 3 weeks versus 10 mg/kg every 3 weeks; (3) a randomized trial of IPI-N patients testing PEM 1 mg/kg every 3 weeks versus 10 mg/kg every 3 weeks (n = 103); and (4) a randomized trial in 244 IPI-N and IPI-T patients testing PEM doses of 10 mg/kg every 3 weeks versus 10 mg/kg every 2 weeks. Thus, the pooled analysis had a total of 655 patients (IPI-T, n = 342; IPI-N, n = 313).
“This very large trial contained multiple randomized and one nonrandomized cohort, so we have the power to answer many questions about programmed cell death-1 (PD-1), including whether or notBRAFmutations confer any reduced response or if upfront treatment helps overall response rate (ORR),” Daud said in an interview withTargeted Oncology. “We also are able to show that patients with lung metastasis benefit much more than [those with] M1a or M1c melanoma and that the dose and schedule have a limited effect on the response rate,” he said.
Patient Characteristics
Most patients were male, and the median age was 61 years (18-94 years). Approximately 25% of patients hadBRAFv600-mutant (MT) status, and 75% hadBRAFv600wild-type (WT) status. According to Daud, elevated lactose dehydrogenase (LDH) levels were present in 38% of patients, 78% of patients had M1c disease, and 75% of patients had received prior systemic therapies.
Safety in the Total Population
The mean duration of exposure to PEM was 15 months (8-29 months). As of May 2015, approximately 180 patients (27%) were still receiving treatment with PEM. The incidence of any grade treatment-related adverse events (AEs) was 82% in the IPI-T group versus 85% in the IPI-N group. The incidence of grade 3 to 4 treatment-related AEs was 14% in each group, and no treatment-related deaths were reported. Only 4% of patients in each group discontinued because of treatment-related AEs.
The most common AEs with an immune etiology were thyroid events occurring as hypothyroidism of any grade in 7.5% of patients, with 0.2% of patients experiencing a grade 3 to 4 event, and as hyperthyroidism of any grade in 2.3% of patients, of whom 0.3% experienced a grade 3 to 4 event. Pneumonitis and colitis of any grade, or grade 3 to 4, were reported by 2.7% and 0.3%, and by 1.7% and 1.1% of patients, respectively.
Efficacy in the Total Population
At 24 months, 70% of patients were still receiving treatment with PEM; 8% (95% confidence interval [CI], 6-11) of patients had a complete response (CR); and 33% (95% CI, 30-37) experienced objective responses. Tumor shrinkage occurred in 71% of patients, with a median change from baseline of 36%. The ORR was significantly higher in the M1b population than in the M1a or M1c groups. Elevated LDH was found to be associated with a lower likelihood of response, and patients with reduced tumor burden experienced higher response rates. There was no significant difference in ORR for the doses of 2 mg/kg or 10 mg/kg.
Median overall survival (OS) and median progression-free survival (PFS) were 22.8 months (95% CI, 19.8-28.7) and 4.4 months (95% CI, 3.1-5.5), respectively. The rates of OS at 12 and 24 months were 66% and 49%, and the rate at 12 months for PFS was 35%.
Efficacy in the First-Line Population
Daud stated that the duration of response was found to be more prolonged in the first-line population, and the median has not yet been reached (2.7+ months - 27.5+ months). The number of patients without progression was 86%, and a median tumor shrinkage of -54% was experienced by 80% of patients.
The Figure shows the results of CR and ORR experienced in the total first-line population and also inBRAFv600-WT andBRAFv600-MT patients.
Figure. Results of CR and ORR experienced in the total first-line population and also in patients with BRAFv600-WT and BRAFv600-MT.
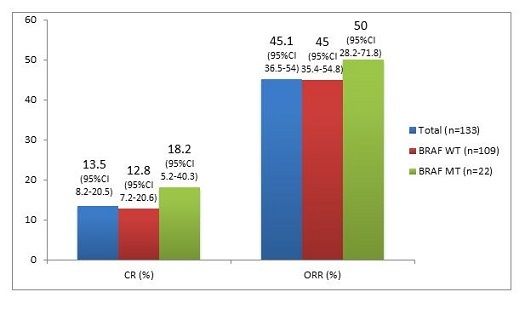
Figure. Results of CR and ORR experienced in the total first-line population and also in patients with BRAFv600-WT and BRAFv600-MT.
Daud, acknowledging that such data were controversial, pointed out that the CR and ORR results in theBRAFv600-MT group were at least as good numerically or better than the data for theBRAFv600-WT group.
Kaplan-Meier estimates of median OS and median PFS (n = 152) were 31.1 months (95% CI, 24-NR) and 13.8 months (95% CI, 6.7-17.4), respectively. The rates for OS at 12 and 24 months were 73% and 60%, respectively, and the rate for PFS at 12 months was 52%.
When asked why patients who were treatment-naïve have better results, Daud said, “This is a great question. There could be several possible mechanisms why later line therapy is not as effective. These include treatment (BRAF)-related changes to tumor, chemotherapy-related immune suppression, adverse selection imposed by treatment failure (IPI refractory patients), as well as other possible effects.”
PD-L1 Expression
Daud also presented data obtained using a programmed cell death ligand 1 (PD-L1) analysis, using data from 67% of the first 411 enrolled patients. Using the Allred Proportional Score (APS, range 1-5), investigators examined the membrane staining of PD-L1 in both tumor and tumor-infiltrating immune cells. The five-step scoring system found that, even at the lowest scores of 0 and 1, some patients experienced responses. At an APS score of 4, approximately 70% of patients experienced responses. There was a correlation between PD-L1 expression and ORR (P<.0001).
There has been much discussion about PD-L1 as a biomarker, and Daud commented, “Certainly, PD-L1 is a biomarker for response. We are still trying to understand what the benefit of PD-L1 is, in terms of patient selection, but unquestionably as PD-L1 expression increases up to APS 4, there is an increased response rate. Also true is that, even at low levels of expression, there are responses and there is a plateau even with high levels of PD-L1 so that there is no further increase beyond 60% to 70% ORRIn other words, other factors aside from PD-L1 are needed for response.”
Conclusion
Daud emphasized the safety and tolerability of PEM, the low incidence of grade 3 to 4 AEs, and the zero incidence of treatment-related deaths. The efficacy was robustly demonstrated in both the total and first-line populations. The duration of response was 28.2 months in the total population and not yet reached in the first-line population. Survival was 49% at 2 years for the total population, and 60% for the first-line population, which also demonstrated a high ORR at 45% and 50% for theBRAFv600WT andBRAFv600MT.
The results support the use of PEM in patients with advanced melanoma regardless of prior therapy.
When asked what the prospects were for combination therapy with PEM, Daud said they were excellent. “Given the relatively low toxicity profile of pembrolizumab, it is a natural combination target. In terms of which agents make sense to combine with PEM, I would say immunologic agents such as intratumoral therapies such as viruses and cytokines, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies, novel checkpoints, chemotherapy….all have a rationale.”
Reference
1. ASCO Meeting Library.http://meetinglibrary.asco.org/content/109620?media=vm. Accessed June 20, 2015.