How Reliable is PD-L1 Expression as a Predictor of Immune Response?
Ovarian cancer is the most lethal of the gynecologic malignancies. Over 22,000 new cases of ovarian cancer are diagnosed each year in the United States, resulting in more than 14,000 deaths per year.
Bradley J. Monk, MD
Ovarian cancer is the most lethal of the gynecologic malignancies. Over 22,000 new cases of ovarian cancer are diagnosed each year in the United States, resulting in more than 14,000 deaths per year. The 5-year survival rate is less than 25% for women with a diagnosis of advanced stage disease (stage III or IV) despite aggressive treatment with surgery and adjuvant chemotherapy. Although more than 80% of patients will respond to initial therapy, epithelial ovarian cancer ultimately recurs in most of them. Recurrence is associated with a poor prognosis because of the eventual development of chemotherapy-resistant disease. Thus, there is a great needand opportunity—to improve ovarian cancer outcomes by understanding the immune milieu of ovarian cancers and harnessing the power of immunotherapy.
While there have been various approaches, from cancer vaccines to adoptive immune-cell therapies, immune checkpoint inhibitors have caused a paradigm shift in cancer treatment (Table 11). The enthusiasm for this approach stems from evidence of complete and long-lasting tumor regression in malignancies that are often refractory to chemotherapy.
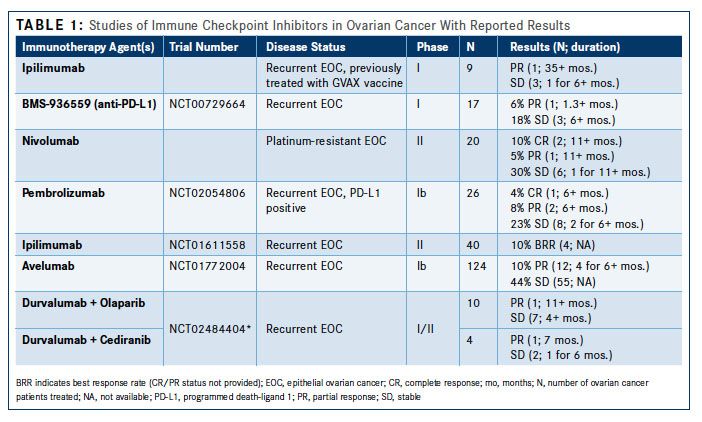
Three principal measures have emerged to predict effective treatment with immune checkpoint inhibitors: (1) accessibility of the tumor by effector immune cells, (2) dominance of the immune checkpoint pathways in suppressing antitumor immunity, and (3) the number of neoantigens, sometimes expressed as mutational burden. The first is frequently defined by the presence of tumor-infiltrating lymphocytes (TIL) or the ratio of effector immune cells (Table 21). The second principle is less well defined, as no accurate biomarker has been identified, although multiple approaches are being evaluated. Expression of PD-L1 on tumor cells has been suggested as a predictive biomarker to identify cancers that may be more responsive to PD-1/PD-L1 inhibitors.
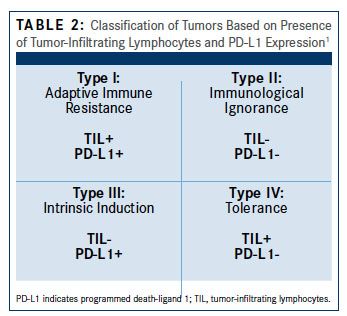
Evidence of the importance of the local tumor immune microenvironment in ovarian cancer emerged in 2003 when it was shown that infiltration of treatment-naïve tumors with T cells was associated with significantly improved median progression-free survival (22.4 vs 5.8 months, P <.001) and overall survival (50.3 vs 18 months, P <.001) compared with tumors with no T cells present.2 However, we have since learned not only that the presence of T cells is important but also that the type of T cells also influences outcomes. The proportion of regulatory T cells (Tregs) in the tumor negatively affects clinical outcomes and was a predictor of increased risk of death in a multivariate analysis. Multiple studies have since confirmed that the ratio of immune suppressive to immune effector cells within ovarian tumors is associated with clinical outcome. Immune responses to ovarian cancer appear to vary by histologic subtype, with high-grade serous cancers most likely to be associated with a prognostically favorable tumor-infiltrating lymphocyte response. Classification of different histologic subtypes of ovarian cancers based on TIL and PD-L1 revealed that type I patterns were more common in high-grade serous cancers, while type IV patterns predominated in other histologic subtypes.
Hamanishi and colleagues first reported that high expression of PD-L1 on ovarian cancer cells was associated with poorer outcomes.3 The 5-year survival rate for patients with high- versus low-expressing PD-L1 tumors was 52.6 ± 7.7% versus 80.2 ± 8.9% (P = .016), respectively. PD-L2 expression was also associated with poorer outcomes, but that was not statistically significant. High expression of PD-L1 on ovarian cancer cells was associated with reduced infiltration of cytotoxic T lymphocytes into tumors, suggesting that PD-L1 expression promotes an immunosuppressive microenvironment by inhibiting T-cell infiltration. Both PD-L1 expression and TIL were independent prognostic factors, although PD-L1 expression was inversely correlated with survival. In preclinical models, PD-L1 expression can be induced by interferon gamma (often produced by TILs) and administration of chemotherapy, suggesting a balance that maintains an immune suppressive environment. PD-1/ PD-L1 blockade causes regression of ovarian tumors in a syngeneic ovarian cancer mouse model, further validating the importance of this regulatory pathway.
PD-L1 expression is not limited to tumor cells and has been reported on immune cells including antigen-presenting cells, T cells, and B cells. A recent study showed that PD-L1 expression is predominantly expressed by macrophages in ovarian cancer rather than on the ovarian cancer cells themselves; in this context, macrophage-associated PD-L1 expression was a marker of favorable prognosis. The differences between this study and the one above may be due to the differences in the antibodies used, but they reflect the developing understanding of PD-L1 expression and its prognostic role in ovarian cancer. PD-L1 expression may be a marker of a tumor poised to respond to immune stimulatory effects of chemotherapy; or, perhaps because PD-L1 may suppress the activity of immune-suppressive immune cells (ie, Tregs), PD-L1 expression on immune cells could tip the balance toward a more favorable immune microenvironment. Thus, evaluating PD-L1 expression on tumor cells in isolation is not sufficient to predict immune response and efficacy of immune checkpoint blockade in ovarian cancer.
References:
- Teng MW, Khanna R, Smyth MJ. Checkpoint immunotherapy: picking a winner. Cancer Discov. 2016;6(8):818-820. doi: 10.1158/2159-8290.CD-16-0694.
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003:348(3):203-213. doi: 10.1056/NEJMoa020177.
- Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of antiPD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015-4022. doi: 10.1200/JCO.2015.62.3397.