Searching for the Key to Trastuzumab Cardiotoxicity in HER2+ Breast Cancer
Treatment with trastuzumab (Herceptin) has shown such a benefit in the treatment of patients with HER2-positive breast cancer that it is now the standard of care despite its association with cardiotoxicity. As such, researchers have taken to searching for ways to lessen or prevent the incidence of cardiac events in trials with trastuzumab.
Treatment with trastuzumab (Herceptin) has shown such a benefit in the treatment of patients with HER2-positive breast cancer that it is now the standard of care despite its association with cardiotoxicity. As such, researchers have taken to searching for ways to lessen or prevent the incidence of cardiac events in trials with trastuzumab.
History of Cardiotoxicity Events With Trastuzumab and Anthracyclines
1
When trastuzumab was first used to treat patients with breast cancer, incidents of severe cardiotoxicity were noted; nonetheless, the drug was approved by the FDA in 1998 because of the benefit it had on patient outcomes. In the pivotal trastuzumab trial by Slamon et al, patients with HER2-positive metastatic breast cancer treated with trastuzumab plus chemotherapy achieved a median overall survival (OS) of 25.1 versus 20.3 months for those treated with standard chemotherapy alone (P= .01) and demonstrated a 20% reduction in the risk of death.
Despite these positives, heart failure of New York Heart Association class 3 or 4 was observed in 27% of patients receiving an anthracycline, cyclophosphamide, and trastuzumab; in 13% of patients receiving paclitaxel and trastuzumab; and in 8% of patients receiving an anthracycline and cyclophosphamide.
2
Further trials have shown that trastuzumab treatment combined with an anthracycline leads to an increased risk of heart failure. In a meta-analysis of 29,598 patients with breast cancer treated with trastuzumab across 58 studies, the incidence of severe cardiotoxicity noted in patients with early breast cancer not treated with an anthracycline was 0.92%. This increased to 2.9% with anthracycline treatment.
In the meta-analysis, 3% (95% CI, 2.41-3.64) of all patients experienced severe cardiotoxicity. The incidence rate was 2.62% (95% CI, 1.97-3.35) in patients with early breast cancer and 3.14% (95% CI, 2.12-4.37) in patients with metastatic breast cancer. The proportion also increased after the first year of treatment. “Following trastuzumab initiation, approximately 3 in 100 patients develop severe cardiotoxicity after 2 years,” the investigators noted in the abstract. However, as clinical trials usually exclude patients with a history of cardiovascular disease, the incidence of heart failure could be even higher in a real-life setting.
3
4
5
The presence of HER2 receptors on cardiac myocytes signals a possible mechanism of trastuzumab-associated cardiotoxicity, although the exact causes are still unconfirmed. Researcher Matthew Zeglinski, PhD, suggested that without normal HER2 function there could be an accumulation of reactive oxygen species that leads to cardiac dysfunction.Another report suggests that trastuzumab causes congestive heart failure by increasing systemic vascular resistance and myocardial workload while simultaneously reducing microvascular coronary blood flow.Myocytes that are already damaged are unable to recover in the presence of the monoclonal antibody, which could suggest why the incidence of cardiotoxicity is increased when trastuzumab is given concurrently or following treatment with an anthracycline.
6
“Controversy remains with regard to discontinuing the use of anthracyclines altogether in HER2-positive disease, and therefore, we should endeavor to develop guidance to maximize the cardiac safety of the anthracycline/trastuzumab combination,” Helena M. Earl, MBBS, et al said in a report on trastuzumab cardiac events.
7
In the BCIRG-006 trial, investigators compared a nonanthracycline regimen with trastuzumab against an anthracycline regimen with or without trastuzumab. The OS for patients receiving doxorubicin (Adriamycin)/cyclophosphamide followed by docetaxel [AC-T] and trastuzumab was 92%; for patients receiving docetaxel/ carboplatin plus trastuzumab, 91%; and for patients receiving AC-T without trastuzumab, 87%.Rates of congestive heart failure and cardiac dysfunction were higher among patients receiving the AC-T plus trastuzumab regimen compared with those in the nonanthracycline and trastuzumab arm (P<.001).
Perhaps in the future, concurrent anthracycline use could be eliminated, which may diminish the incidence of heart failure. In the meantime, patients with breast cancer still receive anthracyclines in the adjuvant setting.
Duration Variation for Reduced Cardiotoxicity
The cardiotoxic effects of trastuzumab are not affected by dosage, but the duration of treatment could have an effect on the rate of cardiotoxicity in patients with HER2-positive breast cancer.
8
The HERA trial explored 1 versus 2 years of adjuvant trastuzumab treatment in a large trial of more than 5000 women with HER2-positive early breast cancer. The additional year of treatment with trastuzumab did not provide any additional clinical benefit; however, more cardiac events were noted in the 2-year arm compared with the 1-year arm.Decreases in left ventricular ejection fraction (LVEF) were noted in 7.2% of patients in the 2-year group versus 4.1% in the 1-year group.
6
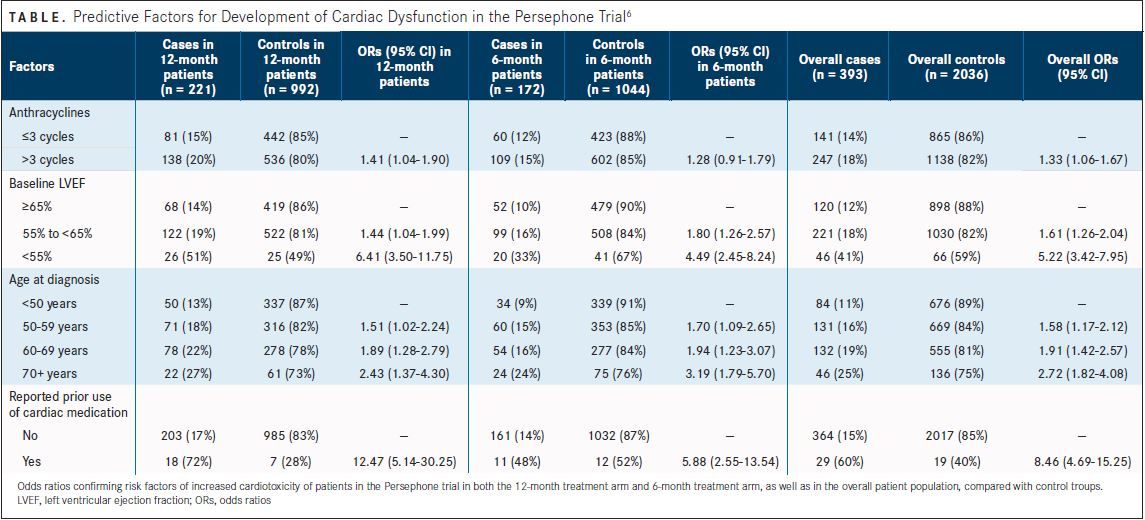
Following results of the HERA trial, the Persephone trial randomized patients to either 6 months or 1 year of trastuzumab. Fewer cardiac events were noted in patients receiving only 6 months of adjuvant trastuzumab than those in the 12-month treatment group.In the 6-month group, 1 cardiac death (0.08%) was noted, 105 patients (9% of 1216) experienced clinical cardiac dysfunction, and 117 (9% of 1249) had LVEF below 50%. In the 12-month group there were 3 cardiac deaths (0.2%), 140 patients (12% of 1214) had clinical cardiac dysfunction, and 146 patients (12% of 1251) had low LVEF. An analysis showed that more cardiac events occurred between months 7 and 12, with 6% of patients in the 12-month arm experiencing a cardiac event versus 3% in the 6-month arm (P= .0002).
(TABLE)
The Persephone trial also confirmed the risk factors for greater cardiotoxicity incidence that were found in the HERA trial, including older age, prior cardiovascular disease, lower baseline LVEF, and more anthracycline treatment.
If 6 months of trastuzumab treatment was found to be statistically noninferior to 12 months of treatment in the Persephone trial, the standard of care could shift to 6 months of treatment, which could diminish the risk of cardiotoxicity, the authors noted in their study.
Prophylactic Heart Failure Treatment
As angiotensin-converting enzyme (ACE) inhibitors and betablockers are recommended agents for treating heart failure, it was hypothesized that cardiotoxicity could be prevented in patients receiving trastuzumab treatment with the use of an ACE inhibitor or beta-blocker.
9
In the MANTICORE 101-Breast trial, patients with HER2-positive early breast cancer were randomized to receive perindopril, bisoprolol (Zebeta), or placebo during trastuzumab treatment. Although cardiac dysfunction was less common in the experimental arms compared with the placebo group after cycle 4 with trastuzumab (P= .02), this was not the case after cycle 17.Overall, the study failed to show that cardiac events could be prevented with either perindopril or bisoprolol.
10
Candesartan (Atacand), an angiotensin receptor blocker, and metoprolol (Lopressor), a beta-blocker, were investigated in the PRADA trial to prevent a decline in LVEF for patients with early breast cancer receiving anthracycline-containing chemotherapy with or without trastuzumab and radiation. In the candesartan group, the rate of decline in LVEF was 0.8 (95% CI, -0.4 to 1.9) compared with 2.6 (95% CI, 1.5-3.8) in the placebo group (P= .026).Metoprolol provided no clinical benefit toward LVEF decline.
Neither the MANTICORE 101 nor the PRADA study included patients with any serious cardiovascular history.
11
The cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer from the American Society of Clinical Oncology suggest that by validating the parameters predictive of cardiotoxicity with trastuzumab raised in the HERA trial age, low baseline LVEF, cardiovascular disease history, and anthracycline exposure—patients at greater risk for experiencing low LVEF or congestive heart failure could be identified and protected.
Although the best methods for preventing or reducing cardiovascular risk from treatment with trastuzumab in patients with HER2-positive early breast cancer have yet to be confirmed, trastuzumab remains the standard of care for these patients. As such, trastuzumab-associated cardiotoxicity will continue to be a concern going forward as researchers search for new ways to optimize treatment.
References:
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2.N Engl J Med.2001;344(11):783-792.
- Mantarro S, Rossi M, Bonifazi M, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer.Intern Emerg Med.2016;11(1):123-140. doi:10.1007/s11739-015-1362-x.
- Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: a ‘dual-hit’.Exp Clin Cardiol.2011;16(3):70-74.
- Sandoo A, Kitas GD, Carmichael AR. Breast cancer therapy and cardiovascular risk: focus on trastuzumab.Vasc Health Risk Manag.2015;11:223-228. doi:10.2147/VHRM.S69641.
- Ewer MS, Ewer SM. Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction.J Clin Oncol.2010;28(25):3901-3904. doi:10.1200/JCO.2010.30.6274.
- Earl HM, Vallier A-L, Dunn J, et al. Trastuzumab-associated cardiac events in the Persephone trial.Br J Cancer.2016;115(12):1462-1470. doi:10.1038/bjc.2016.357.
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer.N Engl J Med.2011;365(14):1273-1283. doi:10.1056/NEJMoa0910383.
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al; Herceptin Adjuvant (HERA) Trial Study Team. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial.Lancet.2013;382(9897):1021-1028. doi:10.1016/S0140-6736(13)61094-6.
- Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity [published online November 28, 2016].J Clin Oncol.2016. doi:10.1200/JCO.2016.68.7830.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol.Eur Heart J.2016;37(21):1671-1680. doi:10.1093/eurheartj/ehw022.
- Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in earlystage breast cancer: getting to the heart of the matter.J Clin Oncol.2016;34(10):1030-1033. doi:10.1200/ JCO.2015.64.5515.
