Guidelines Evolving for the Treatment of Bone Metastases in CRPC
Androgen deprivation therapy (ADT) has been the standard of care for patients with advanced prostate cancer; however, a majority of patients on hormonal therapy become unresponsive to treatment after an initial response.
Guidelines Evolving for the Treatment of Bone Metastases in CRPC
Androgen deprivation therapy (ADT) has been the standard of care for patients with advanced prostate cancer; however, a majority of patients on hormonal therapy become unresponsive to treatment after an initial response.1Castration-resistant prostate cancer (CRPC) is partially identified by rising levels of prostate-specific antigen (PSA) despite chemical castration, and more than 90% of patients with CRPC develop bone metastases2(FIGURE 1).
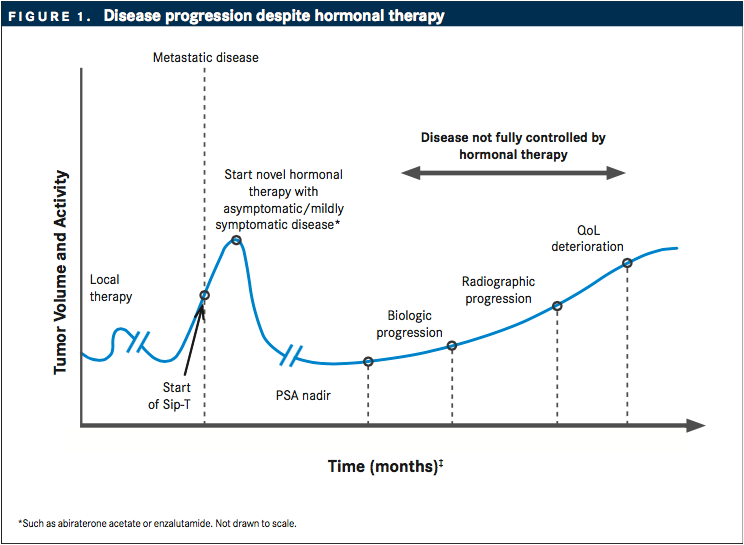
The metastatic CRPC (mCRPC) treatment portfolio is expanding rapidly. Numerous broad-spectrum and targeted therapies undergoing clinical testing are impacting overall treatment options for patients with CRPC. After approval of docetaxel in 2004, five new agents have been approved by the FDA to treat CRPC: abiraterone acetate (Zytiga), enzalutamide (Xtandi), cabazitaxel (Jevtana), radium-223 (Xofigo), and sipuleucel-T (Provenge).3
NCCN Prostate Cancer Guidelines
The NCCN’s clinical practice guidelines, a reference for standard of care in the United States, are updated regularly to address new findings. The guidelines represent a consensus statement that is compiled using data from a variety of clinical trials, with the highest level of evidence, labeled category 1, provided by multi-national, multi-institutional, randomized controlled trials.
While most of the updates in 2016 focused on imaging, radiation therapy, diagnostics, and other changes, the preceding updates centered on the use of systemic agents. In first-line therapy for CRPC patients with no visceral metastases, the following therapies have all received a category 1 listing:
- Enzalutamide
- Abiraterone acetate with prednisone
- Docetaxel with prednisone
- Radium-223 for men with symptomatic bone metastases
Enzalutamide for men with asymptomatic or mildly symptomatic bone metastases
This recommendation was based on results from the multinational, double-blind, randomized phase III PREVAIL trial, which examined the effect of enzalutamide administration in asymptomatic or mildly symptomatic chemotherapy-naive men with mCRPC. The trial showed that treatment with enzalutamide significantly improved OS (30% reduction in risk of death) and radiographic PFS (81% reduction in risk of radiographic PFS) in men with chemotherapynaive mCRPC.4
Abiraterone with prednisone for men with asymptomatic or mildly symptomatic mCRPC
This recommendation was based on interim analyses of the COU-AA-302 trial. In this placebo-controlled, double-blind, randomized phase III study on 1088 asymptomatic or mildly symptomatic patients with chemotherapy-naive PC, abiraterone acetate plus prednisone significantly improved radiographic progression-free survival (PFS) compared with placebo plus prednisone. OS was significantly longer in the abiraterone acetate group than in the placebo group (34.7 vs 30.3 months).5
Docetaxel with prednisone for hormone-naive mCRPC
Changes to the NCCN guidelines also recognize the results from CHAARTED trial showing docetaxel can be repurposed in metastatic CRPC. In this study, the median OS was 13.6 months longer with the combination of androgen-deprivation therapy (ADT) plus docetaxel than with ADT alone, and in men with high-volume metastatic disease, median OS was 17.0 months longer.6
Radium-223 for men with symptomatic bone metastases
This treatment is based on results from the ALSYMPCA phase III study in patients with CRPC with symptomatic bone metastases who received docetaxel, who were unfit to receive docetaxel or declined docetaxel. Study interim analysis on 921 patients showed improved OS benefit (median of 14.9 months versus 11.3). Other phase I-IIa studies are ongoing to study the use of radium-223 in combination with docetaxel for mCRPC.7
AUA CRPC Guideline
The American Urological Association (AUA) guideline focuses on 6 “index patients” ranging from men with asymptomatic, nonmetastatic CRPC to those with symptomatic, metastatic disease with a poor performance status and previous exposure to docetaxel.
The AUA guideline was introduced in 2013 in response to the multitude of newly approved therapies for men with CRPC. The index patient guideline design stresses the importance of knowing exactly where the patient fits, which requires adequate history taking, patient evaluation, and focus on symptoms. Overall, there are 22 guideline statements.
- Index Patient 1 (asymptomatic non-metastatic CRPC):Consider observation, ADT, first-generation anti-androgens and androgen synthesis inhibitors. Do not consider: chemotherapy or immunotherapy
- Index Patient 2 (asymptomatic or minimally symptomatic, mCRPC without prior docetaxel):Consider abiraterone, enzalutamide, docetaxel, sipuleucel-T or first-generation hormonal agents
- Index Patient 3 (symptomatic, mCRPC with good PS and no prior docetaxel):Consider abiraterone, enzalutamide, docetaxel, radium-223, and firstgeneration hormonal agents
- Index Patient 4 (symptomatic, mCRPC with poor PS and no prior docetaxel):Consider abiraterone, enzalutamide, first-generation hormonal agents, docetaxel, and radium-223
- Index Patient 5 (symptomatic, mCRPC with good PS and prior docetaxel):Consider abiraterone, cabazitaxel, enzalutamide, additional docetaxel, radium-223, and ketoconazole, if other options are unavailable
- Index Patient 6 (symptomatic, mCRPC with poor PS and prior docetaxel):palliative care, abiraterone, enzalutamide, and ketoconazole or radionuclide
In addition to the index patients, the guideline also issued two statements on bone health, namely prevention using supplemental calcium and vitamin D or with denosumab (Xgeva) or zoledronic acid (Zometa).
ESMO Magnitude of Clinical Benefit Scale
The European Society for Medical Oncology (ESMO) is developing a validated and reproducible tool to assess the magnitude of clinical benefit of anticancer interventions, the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS), based on initial recommendations from 276 members of the ESMO faculty and a team of 51 expert biostatisticians.
With this scoring tool, ESMO aims to highlight treatments that provide substantial improvements to the duration of survival and/or the quality of life (QoL) of cancer patients and distinguish them from treatments where benefits are more modest, limited, or marginal. ESMO intends to apply this scale prospectively to each new anticancer drug/intervention that will be European Medicines Agency (EMA) approved. Drugs or treatment interventions that obtain the highest scores on the scale will be highlighted in the ESMO guidelines with the aim of prompting rapid endorsement by health authorities across the European Union.9
ALSYMPCA trial using radium-223 with chemotherapy or radiotherapy treatment scored 5, the highest score. Abiraterone with or without prednisone and enzalutamide versus placebo treatments scored next highest, 4. The Tropic trial that utilized cabazitaxel and prednisone versus mitoxantrone and prednisone came at the bottom of the list with a score of 2. Based on this analysis, radium-223 demonstrated the highest clinical benefit in patients with mCRPC.
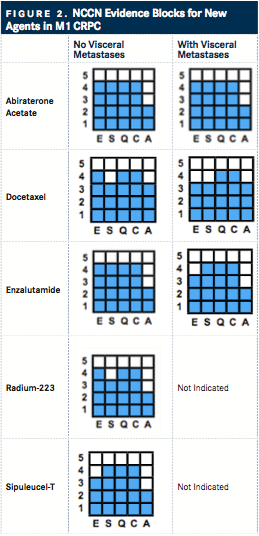
NCCN Evidence Blocks
Similar to the ESMO scale, the NCCN evidence blocks are guidelines for treatment providers to effectively collaborate with their patients in the Unites States. Levels of evidence from 1 to 5 are presently based on five elements: efficacy (E), safety (S), quality (Q) and consistency (C) of evidence, and the affordability (A).
The NCCN Evidence Blocks are not alone; however, other tools lack the flexibility to allow comparisons of drugs evaluated in different trials and does not allow assessments of the range of interventions possible across multiple disease scenarios. Additionally, look beyond costs provides a more thorough picture on the true value of the treatment.
For prostate cancer, this work has already been completed for Version 2.2016 of the NCCN Prostate Cancer Guideline. For those with CRPC, each therapy throughout the NCCN treatment algorithm has been ranked, based on the 5 criteria in that setting (for those in the frontline M1 CRPC space, see FIGURE 2).
The evidence blocks have been evolving, since their initial introduction at the 2015 NCCN 20th Annual Conference. The goal for NCCN is to incorporate the blocks into all of its systemic therapy guidelines by 2017.

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More